Bacterial Topography of the Healthy Human Lower Respiratory Tract
- PMID: 28196961
- PMCID: PMC5312084
- DOI: 10.1128/mBio.02287-16
Bacterial Topography of the Healthy Human Lower Respiratory Tract
Abstract
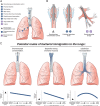
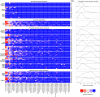
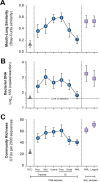
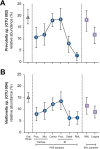
Although culture-independent techniques have refuted lung sterility in health, controversy about contamination during bronchoscope passage through the upper respiratory tract (URT) has impeded research progress. We sought to establish whether bronchoscopic sampling accurately reflects the lung microbiome in health and to distinguish between two proposed routes of authentic microbial immigration, (i) dispersion along contiguous respiratory mucosa and (ii) subclinical microaspiration. During bronchoscopy of eight adult volunteers without lung disease, we performed seven protected specimen brushings (PSB) and bilateral bronchoalveolar lavages (BALs) per subject. We amplified, sequenced, and analyzed the bacterial 16S rRNA gene V4 regions by using the Illumina MiSeq platform. Rigorous attention was paid to eliminate potential sources of error or contamination, including a randomized processing order and the inclusion and analysis of exhaustive procedural and sequencing control specimens. Indices of mouth-lung immigration (mouth-lung community similarity, bacterial burden, and community richness) were all significantly greater in airway and alveolar specimens than in bronchoscope contamination control specimens, indicating minimal evidence of pharyngeal contamination. Ecological indices of mouth-lung immigration peaked at or near the carina, as predicted for a primary immigration route of microaspiration. Bacterial burden, diversity, and mouth-lung similarity were greater in BAL than PSB samples, reflecting differences in the sampled surface areas. (This study has been registered at ClinicalTrials.gov under registration no. NCT02392182.)IMPORTANCE This study defines the bacterial topography of the healthy human respiratory tract and provides ecological evidence that bacteria enter the lungs in health primarily by microaspiration, with potential contribution in some subjects by direct dispersal along contiguous mucosa. By demonstrating that contamination contributes negligibly to microbial communities in bronchoscopically acquired specimens, we validate the use of bronchoscopy to investigate the lung microbiome.
Copyright © 2017 Dickson et al.
Figures
References
-
- Cotran RS, Kumar V, Collins T, Robbins SL. 1999. Robbins pathologic basis of disease, 6th ed. Saunders, Philadelphia, PA.
-
- Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, Jablonski K, Kleerup E, Lynch SV, Sodergren E, Twigg H, Young VB, Bassis CM, Venkataraman A, Schmidt TM, Weinstock GM, Lung HIV Microbiome Project . 2013. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 187:1067–1075. doi: 10.1164/rccm.201210-1913OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

