Glutathionylation of Pea Chloroplast 2-Cys Prx and Mitochondrial Prx IIF Affects Their Structure and Peroxidase Activity and Sulfiredoxin Deglutathionylates Only the 2-Cys Prx
- PMID: 28197170
- PMCID: PMC5283164
- DOI: 10.3389/fpls.2017.00118
Glutathionylation of Pea Chloroplast 2-Cys Prx and Mitochondrial Prx IIF Affects Their Structure and Peroxidase Activity and Sulfiredoxin Deglutathionylates Only the 2-Cys Prx
Abstract
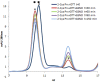
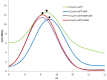
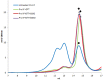
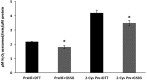
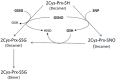
Together with thioredoxins (Trxs), plant peroxiredoxins (Prxs), and sulfiredoxins (Srxs) are involved in antioxidant defense and redox signaling, while their regulation by post-translational modifications (PTMs) is increasingly regarded as a key component for the transduction of the bioactivity of reactive oxygen and nitrogen species. Among these PTMs, S-glutathionylation is considered a protective mechanism against overoxidation, it also modulates protein activity and allows signaling. This study explores the glutathionylation of recombinant chloroplastic 2-Cys Prx and mitochondrial Prx IIF from Pisum sativum. Glutathionylation of the decameric form of 2-Cys Prx produced a change in the elution volume after FPLC chromatography and converted it to its dimeric glutathionylated form, while Prx IIF in its reduced dimeric form was glutathionylated without changing its oligomeric state. Mass spectrometry demonstrated that oxidized glutathione (GSSG) can glutathionylate resolving cysteine (Cys174), but not the peroxidatic equivalent (Cys52), in 2-Cys Prx. In contrast, GSSG was able to glutathionylate both peroxidatic (Cys59) and resolving (Cys84) cysteine in Prx IIF. Glutathionylation was seen to be dependent on the GSH/GSSG ratio, although the exact effect on the 2-Cys Prx and Prx IIF proteins differed. However, the glutathionylation provoked a similar decrease in the peroxidase activity of both peroxiredoxins. Despite growing evidence of the importance of post-translational modifications, little is known about the enzymatic systems that specifically regulate the reversal of this modification. In the present work, sulfiredoxin from P. sativum was seen to be able to deglutathionylate pea 2-Cys Prx but not pea Prx IIF. Redox changes during plant development and the response to stress influence glutathionylation/deglutathionylation processes, which may represent an important event through the modulation of peroxiredoxin and sulfiredoxin proteins.
Keywords: 2-Cys peroxiredoxin; glutathione redox state; glutathionylation; peroxiredoxin IIF; post-translational modification; reactive nitrogen species; reactive oxygen species; sulfiredoxin.
Figures



Similar articles
-
Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin.J Biol Chem. 2009 Aug 28;284(35):23364-74. doi: 10.1074/jbc.M109.021394. Epub 2009 Jun 27. J Biol Chem. 2009. PMID: 19561357 Free PMC article.
-
Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators.Antioxid Redox Signal. 2012 Mar 15;16(6):506-23. doi: 10.1089/ars.2011.4260. Antioxid Redox Signal. 2012. PMID: 22114845 Free PMC article. Review.
-
Glutathionylation induces the dissociation of 1-Cys D-peroxiredoxin non-covalent homodimer.J Biol Chem. 2006 Oct 20;281(42):31736-42. doi: 10.1074/jbc.M602188200. Epub 2006 Aug 17. J Biol Chem. 2006. PMID: 16916801
-
The contribution of NADPH thioredoxin reductase C (NTRC) and sulfiredoxin to 2-Cys peroxiredoxin overoxidation in Arabidopsis thaliana chloroplasts.J Exp Bot. 2015 May;66(10):2957-66. doi: 10.1093/jxb/eru512. Epub 2015 Jan 5. J Exp Bot. 2015. PMID: 25560178 Free PMC article.
-
Multiple functions of 2-Cys peroxiredoxins, I and II, and their regulations via post-translational modifications.Free Radic Biol Med. 2020 May 20;152:107-115. doi: 10.1016/j.freeradbiomed.2020.02.028. Epub 2020 Mar 7. Free Radic Biol Med. 2020. PMID: 32151745 Review.
Cited by
-
Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases.Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C304-C327. doi: 10.1152/ajpcell.00410.2019. Epub 2019 Nov 6. Am J Physiol Cell Physiol. 2020. PMID: 31693398 Free PMC article.
-
Oxidative post-translational modifications of plant antioxidant systems under environmental stress.Physiol Plant. 2025 Jan-Feb;177(1):e70118. doi: 10.1111/ppl.70118. Physiol Plant. 2025. PMID: 39968905 Free PMC article. Review.
-
Reduced GSH Acts as a Metabolic Cue of OPDA Signaling in Coregulating Photosynthesis and Defense Activation under Stress.Plants (Basel). 2023 Nov 1;12(21):3745. doi: 10.3390/plants12213745. Plants (Basel). 2023. PMID: 37960101 Free PMC article.
-
CYP20-3 deglutathionylates 2-CysPRX A and suppresses peroxide detoxification during heat stress.Life Sci Alliance. 2020 Jul 30;3(9):e202000775. doi: 10.26508/lsa.202000775. Print 2020 Sep. Life Sci Alliance. 2020. PMID: 32732254 Free PMC article.
-
Thiol-based Oxidative Posttranslational Modifications (OxiPTMs) of Plant Proteins.Plant Cell Physiol. 2022 Jul 14;63(7):889-900. doi: 10.1093/pcp/pcac036. Plant Cell Physiol. 2022. PMID: 35323963 Free PMC article. Review.
References
-
- Barranco-Medina S., Krell T., Bernier-Villamor L., Sevilla F., Lázaro J. J., Dietz K. J. (2008). Hexameric oligomerization of mitocondrial peroxiredoxin PrxIIF and formation of an ultrahigh affinity complex with its electron donor thioredoxin Trx-o. J. Exp. Bot. 59 3259–3269. 10.1093/jxb/ern177 - DOI - PMC - PubMed
-
- Barranco-Medina S., López-Jaramillo F. J., Bernier-Villamor L., Sevilla F., Lázaro J. J. (2006). Cloning, overexpression, purification and preliminary crystallographic studies of a mitochondrial type II peroxiredoxin from Pisum sativum. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 62 696–698. 10.1107/S1744309106023451 - DOI - PMC - PubMed
-
- Beer S. M., Taylor E. R., Brown S. E., Dahm C. C., Costa N. J., Runswick M. J., et al. (2004). Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant defense. J. Biol. Chem. 279 47939–47951. 10.1074/jbc.M408011200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

