Therapeutic Effects of Fermented Flax Seed Oil on NC/Nga Mice with Atopic Dermatitis-Like Skin Lesions
- PMID: 28197211
- PMCID: PMC5288556
- DOI: 10.1155/2017/5469125
Therapeutic Effects of Fermented Flax Seed Oil on NC/Nga Mice with Atopic Dermatitis-Like Skin Lesions
Abstract
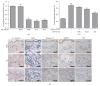
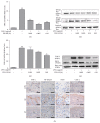
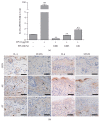
Background. Atopic Dermatitis (AD) is one of the most common chronic inflammatory skin diseases. Objective. This experiment aimed to study the effects of Fermented Flax Seed Oil (FFSO) on symptoms such as redness, eczema, and pruritus induced by AD. Materials and Methods. AD-induced NC/Nga mice were used to observe the immunological and therapeutic effects of FFSO on skin in vivo. Raw 264.7 cells were used to investigate the effects of FFSO in cells. Fc receptor expression and concentration of beta-hexosaminidase were measured. Nitric oxide assay, Western blotting, real-time PCR, image analysis, and statistical analysis were performed in vitro. Results. In the immunohistochemical results, p-ERK 1/2 expression decreased, fibrogenesis strongly increased, and distribution reduction is observed. Distribution of IL-4-positive cells in the corium near the basal portion of the epithelium in the AT group was reduced. FFSO treatment reduced the number of cells showing NF-κB p65 and iNOS expression. The level of LXR in the AT group was higher than that in the AE group, and elevation of PKC expression was significantly reduced by FFSO treatment. Conclusion. FFSO could alleviate symptoms of AD such as epithelial damage, redness, swelling, and pruritus.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

