Herbal Compound "Jiedu Huayu" Reduces Liver Injury in Rats via Regulation of IL-2, TLR4, and PCNA Expression Levels
- PMID: 28197212
- PMCID: PMC5288544
- DOI: 10.1155/2017/9819350
Herbal Compound "Jiedu Huayu" Reduces Liver Injury in Rats via Regulation of IL-2, TLR4, and PCNA Expression Levels
Abstract
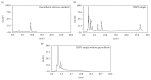
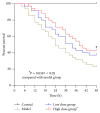
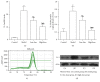
Aim of the Study. To investigate the preventative effects of Jiedu Huayu (JDHY) on D-galactosamine (D-GalN) and lipopolysaccharide-induced acute liver failure (ALF) and to evaluate the possible mechanisms of action. Materials and Methods. ALF was induced in Wistar rats by administrating D-GalN (900 mg/kg) and lipopolysaccharide (10 μg/kg). After treatment with JDHY granules, the levels of blood alanine aminotransferase, aspartate aminotransferase, total bilirubin, and prothrombin time were determined. Proliferating cell nuclear antigen was detected by immunohistochemistry staining. The expression of interleukin-2 (IL-2) and toll-like receptor 4 (TLR4) was examined by fluorescence quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot. Results. JDHY treatment dramatically improved liver function and increased survival rates in an ALF model in rats. We observed a decrease in IL-2 and TLR4 expression following treatment with JDHY in liver cells from ALF rats using qRT-PCR and Western blot analysis. Conclusion. We hypothesize that the therapeutic potential of JDHY for treating ALF is due to its modulatory effect on the suppression of inflammation and by promoting hepatocyte regeneration. Our results contribute towards validation of the traditional use of JDHY in the treatment of liver disease.
Conflict of interest statement
The authors declare that no conflict of interests exists.
Figures



References
-
- Nie X.-H., Han T., Ha F.-U., et al. Comparison of the effects of the pretreatment and treatment with RhIL-11 on acute liver failure induced by D-galactosamine. European Review for Medical and Pharmacological Sciences. 2014;18(8):1142–1150. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

