Identification and Mechanistic Evaluation of Hemozoin-Inhibiting Triarylimidazoles Active against Plasmodium falciparum
- PMID: 28197312
- PMCID: PMC5304302
- DOI: 10.1021/acsmedchemlett.6b00416
Identification and Mechanistic Evaluation of Hemozoin-Inhibiting Triarylimidazoles Active against Plasmodium falciparum
Abstract
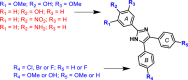
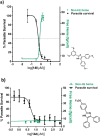
In a previous study, target based screening was carried out for inhibitors of β-hematin (synthetic hemozoin) formation, and a series of triarylimidazoles were identified as active against Plasmodium falciparum. Here, we report the subsequent synthesis and testing of derivatives with varying substituents on the three phenyl rings for this series. The results indicated that a 2-hydroxy-1,3-dimethoxy substitution pattern on ring A is required for submicromolar parasite activity. In addition, cell-fractionation studies revealed uncommonly large, dose-dependent increases of P. falciparum intracellular exchangeable (free) heme, correlating with decreased parasite survival for β-hematin inhibiting derivatives.
Keywords: Antimalarial; Plasmodium falciparum; hemozoin; triarylimidazole.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Identification of β-hematin inhibitors in a high-throughput screening effort reveals scaffolds with in vitro antimalarial activity.Int J Parasitol Drugs Drug Resist. 2014 Sep 11;4(3):316-25. doi: 10.1016/j.ijpddr.2014.08.002. eCollection 2014 Dec. Int J Parasitol Drugs Drug Resist. 2014. PMID: 25516843 Free PMC article.
-
A Diverse Range of Hemozoin Inhibiting Scaffolds Act on Plasmodium falciparum as Heme Complexes.ACS Infect Dis. 2021 Feb 12;7(2):362-376. doi: 10.1021/acsinfecdis.0c00680. Epub 2021 Jan 12. ACS Infect Dis. 2021. PMID: 33430579 Free PMC article.
-
Antimalarial drugs inhibiting hemozoin (beta-hematin) formation: a mechanistic update.Life Sci. 2007 Feb 6;80(9):813-28. doi: 10.1016/j.lfs.2006.11.008. Epub 2006 Nov 10. Life Sci. 2007. PMID: 17157328 Review.
-
Hemozoin inhibiting 2-phenylbenzimidazoles active against malaria parasites.Eur J Med Chem. 2018 Nov 5;159:243-254. doi: 10.1016/j.ejmech.2018.09.060. Epub 2018 Sep 28. Eur J Med Chem. 2018. PMID: 30296683 Free PMC article.
-
Targeting the hemozoin synthesis pathway for new antimalarial drug discovery: technologies for in vitro beta-hematin formation assay.Comb Chem High Throughput Screen. 2005 Feb;8(1):63-79. doi: 10.2174/1386207053328101. Comb Chem High Throughput Screen. 2005. PMID: 15720198 Review.
Cited by
-
Benzylic C-H Oxidation: Recent Advances and Applications in Heterocyclic Synthesis.Molecules. 2024 Dec 22;29(24):6047. doi: 10.3390/molecules29246047. Molecules. 2024. PMID: 39770135 Free PMC article. Review.
-
Heme Detoxification in the Malaria Parasite: A Target for Antimalarial Drug Development.Acc Chem Res. 2021 Jun 1;54(11):2649-2659. doi: 10.1021/acs.accounts.1c00154. Epub 2021 May 13. Acc Chem Res. 2021. PMID: 33982570 Free PMC article. Review.
-
The Antagonizing Role of Heme in the Antimalarial Function of Artemisinin: Elevating Intracellular Free Heme Negatively Impacts Artemisinin Activity in Plasmodium falciparum.Molecules. 2022 Mar 8;27(6):1755. doi: 10.3390/molecules27061755. Molecules. 2022. PMID: 35335120 Free PMC article.
-
Insights into structural and physicochemical properties required for β-hematin inhibition of privileged triarylimidazoles.RSC Med Chem. 2019 Dec 16;11(1):85-91. doi: 10.1039/c9md00468h. eCollection 2020 Jan 1. RSC Med Chem. 2019. PMID: 33479606 Free PMC article.
-
Identifying inhibitors of β-haematin formation with activity against chloroquine-resistant Plasmodium falciparum malaria parasites via virtual screening approaches.Sci Rep. 2023 Feb 14;13(1):2648. doi: 10.1038/s41598-023-29273-w. Sci Rep. 2023. PMID: 36788274 Free PMC article.
References
-
- Gamo F.-J.; Sanz L. M.; Vidal J.; de Cozar C.; Alvarez E.; Lavandera J.-L.; Vanderwall D. E.; Green D. V. S.; Kumar V.; Hasan S.; Brown J. R.; Peishoff C. E.; Cardon L. R.; Garcia-Bustos J. F. Thousands of Chemical Starting Points for Antimalarial Lead Identification. Nature 2010, 465, 305–312. 10.1038/nature09107. - DOI - PubMed
-
- Guiguemde W. A.; Shelat A. A.; Bouck D.; Duffy S.; Crowther G. J.; Davis P. H.; Smithson D. C.; Connelly M.; Clark J.; Zhu F.; Jimenez-Diaz M. B.; Martinez M. S.; Wilson E. B.; Tripathi A. K.; Gut J.; Sharlow E. R.; Bathurst I.; Mazouni F. E.; Fowble J. W.; Forquer I.; McGinley P. L.; Castro S.; Angulo-Barturen I.; Ferrer S.; Rosenthal P. J.; DeRisi J. L.; Sullivan D. J. Jr; Lazo J. S.; Roos D. S.; Riscoe M. K.; Phillips M. A.; Rathod P. K.; Van Voorhis W. C.; Avery V. M.; Guy R. K. Chemical Genetics of Plasmodium falciparum. Nature 2010, 465, 311–315. 10.1038/nature09099. - DOI - PMC - PubMed
-
- World Health Organization. Antimalarial Drug Resistance. http://www.who.int/malaria/areas/drug_resistance/overview/en/ (October 2014).
-
- Fidock D. A.; Nomura T.; Talley A. K.; Cooper R. A.; Dzekunov S. M.; Ferdig M. T.; Ursos L. M. B.; Sidhu A. S.; Naude B.; Deitsch K. W.; Su X.; Wootton J. C.; Roepe P. D.; Wellems T. E. Mutations in the P. falciparum Digestive Vacuole Transmembrane Protein PfCRT and Evidence for Their Role in Chloroquine Resistance. Mol. Cell 2000, 6, 861–871. 10.1016/S1097-2765(05)00077-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

