Design and Synthesis of Novel, Selective GPR40 AgoPAMs
- PMID: 28197316
- PMCID: PMC5304298
- DOI: 10.1021/acsmedchemlett.6b00443
Design and Synthesis of Novel, Selective GPR40 AgoPAMs
Abstract
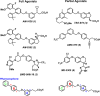
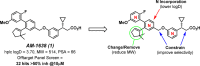
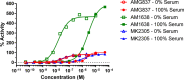
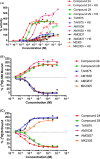
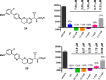
GPR40 is a G-protein-coupled receptor expressed primarily in pancreatic islets and intestinal L-cells that has been a target of significant recent therapeutic interest for type II diabetes. Activation of GPR40 by partial agonists elicits insulin secretion only in the presence of elevated blood glucose levels, minimizing the risk of hypoglycemia. GPR40 agoPAMs have shown superior efficacy to partial agonists as assessed in a glucose tolerability test (GTT). Herein, we report the discovery and optimization of a series of potent, selective GPR40 agoPAMs. Compound 24 demonstrated sustained glucose lowering in a chronic study of Goto Kakizaki rats, showing no signs of tachyphylaxis for this mechanism.
Keywords: FFA1; GPCR; GPR40; agoPAM; chroman; diabetes; insulin secretogogue.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Itoh Y.; Kawamata Y.; Harada M.; Kobayashi M.; Fujii R.; Fukusumi S.; Ogi K.; Hosoya M.; Tanaka Y.; Uejima H.; Tanaka H.; Maruyama M.; Satah R.; Okubo S.; Kizawa H.; Komatsu H.; Matsumura F.; Moguchi Y.; Shinohara T.; Hinuma S.; Fujisawa Y.; Fujino M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. 10.1038/nature01478. - DOI - PubMed
-
- Negoro N.; Sasaki S.; Mikami S.; Ito M.; Suzuki M.; Tsujihata Y.; Ito R.; Harada A.; Takeuchi K.; Suzuki N.; Miyazaki J.; Santou T.; Odani T.; Kanzaki N.; Funami M.; Tanaka T.; Kogame A.; Matsunaga S.; Yasuma T.; Momose Y. Discovery of TAK-875: A potent, selective, and orally bioavailable GPR40 agonist. ACS Med. Chem. Lett. 2010, 1, 290–294. 10.1021/ml1000855. - DOI - PMC - PubMed
-
- Christiansen E.; Due-Hansen M. E.; Urban C.; Merten N.; Pfleiderer M.; Karlsen K. K.; Rasmussen S. S.; Steensgaard M.; Hamacher A.; Schmidt J.; Drewke C.; Petersen R. K.; Kristiansen K.; Ullrich S.; Kostenis E.; Kassak M. U.; Ulven T. Structure activity study of dihydrocinnamic acids and the discovery of the potent FFA1 (GPR40) agonist TUG-469. ACS Med. Chem. Lett. 2010, 1, 345–349. 10.1021/ml100106c. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

