Discovery of VU0467485/AZ13713945: An M4 PAM Evaluated as a Preclinical Candidate for the Treatment of Schizophrenia
- PMID: 28197318
- PMCID: PMC5304297
- DOI: 10.1021/acsmedchemlett.6b00461
Discovery of VU0467485/AZ13713945: An M4 PAM Evaluated as a Preclinical Candidate for the Treatment of Schizophrenia
Abstract
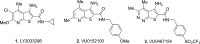
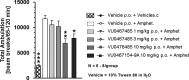
Herein, we report the structure-activity relationships within a series of potent, selective, and orally bioavailable muscarinic acetylcholine receptor 4 (M4) positive allosteric modulators (PAMs). Compound 6c (VU0467485) possesses robust in vitro M4 PAM potency across species and in vivo efficacy in preclinical models of schizophrenia. Coupled with an attractive DMPK profile and suitable predicted human PK, 6c (VU0467485) was evaluated as a preclinical development candidate.
Keywords: Positive allosteric modulator (PAM); VU0467485; muscarinic acetylcholine receptor 4 (M4); schizophrenia.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors are developing M4 PAMs for the treatment of schizophrenia and hold patents on the same.
Figures





References
-
- Bodick N. C.; Offen W. W.; Levey A. I.; Cutler N. R.; Gauthier S. G.; Satlin A.; Shannon H. E.; Tollefson G. D.; Rasmussen K.; Bymaster F. P.; Hurley D. J.; Potter W. Z.; Paul S. M. Effects of Xanomeline, a Selective Muscarinic Receptor Agonist, on Cognitive Function and Behavioral Symptoms in Alzheimer Disease. Arch. Neurol. 1997, 54, 465–473. 10.1001/archneur.1997.00550160091022. - DOI - PubMed
-
- Shekhar A.; Potter W. Z.; Lightfoot J.; Lienemann J.; Dube S.; Mallinckrodt C.; Bymaster F. P.; McKinzie D. L.; Felder C. C. Selective muscarinic receptor agonist Xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 2008, 165, 1033–1039. 10.1176/appi.ajp.2008.06091591. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

