Defective T-cell control of Epstein-Barr virus infection in multiple sclerosis
- PMID: 28197337
- PMCID: PMC5292561
- DOI: 10.1038/cti.2016.87
Defective T-cell control of Epstein-Barr virus infection in multiple sclerosis
Erratum in
-
Erratum: Defective T-cell control of Epstein-Barr virus infection in multiple sclerosis.Clin Transl Immunology. 2017 Jun 16;6(6):e147. doi: 10.1038/cti.2017.25. eCollection 2017 Jun. Clin Transl Immunology. 2017. PMID: 28748090 Free PMC article.
Abstract
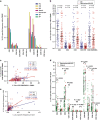
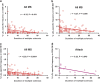
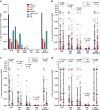
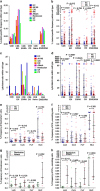
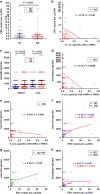
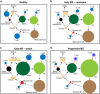
Mounting evidence indicates that infection with Epstein-Barr virus (EBV) has a major role in the pathogenesis of multiple sclerosis (MS). Defective elimination of EBV-infected B cells by CD8+ T cells might cause MS by allowing EBV-infected autoreactive B cells to accumulate in the brain. Here we undertake a comprehensive analysis of the T-cell response to EBV in MS, using flow cytometry and intracellular IFN-γ staining to measure T-cell responses to EBV-infected autologous lymphoblastoid cell lines and pools of human leukocyte antigen (HLA)-class-I-restricted peptides from EBV lytic or latent proteins and cytomegalovirus (CMV), in 95 patients and 56 EBV-seropositive healthy subjects. In 20 HLA-A2+ healthy subjects and 20 HLA-A2+ patients we also analysed CD8+ T cells specific for individual peptides, measured by binding to HLA-peptide complexes and production of IFN-γ, TNF-α and IL-2. We found a decreased CD8+ T-cell response to EBV lytic, but not CMV lytic, antigens at the onset of MS and at all subsequent disease stages. CD8+ T cells directed against EBV latent antigens were increased but had reduced cytokine polyfunctionality indicating T-cell exhaustion. During attacks the EBV-specific CD4+ and CD8+ T-cell populations expanded, with increased functionality of latent-specific CD8+ T cells. With increasing disease duration, EBV-specific CD4+ and CD8+ T cells progressively declined, consistent with T-cell exhaustion. The anti-EBNA1 IgG titre correlated inversely with the EBV-specific CD8+ T-cell frequency. We postulate that defective CD8+ T-cell control of EBV reactivation leads to an expanded population of latently infected cells, including autoreactive B cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004; 350: 1328–1337. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
