Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer
- PMID: 28197389
- PMCID: PMC5283618
- DOI: 10.1080/2162402X.2016.1261779
Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer
Abstract
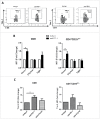
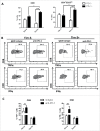
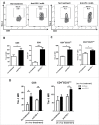
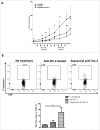
Programmed Death 1 (PD-1) and T cell Ig and mucin domain-3 protein (Tim-3) are immune checkpoint receptors that are expressed on tumor-infiltrating lymphocytes (TIL) in tumor-bearing mice and humans. As anti-PD-1 single agent response rates are only <20% in head and neck squamous cell carcinoma (HNSCC) patients, it is important to understand how multiple inhibitory checkpoint receptors maintain suppressed cellular immunity. One such receptor, Tim-3, activates downstream proliferative pathways through Akt/S6, and is highly expressed in dysfunctional TIL. We observed that PD-1 and Tim-3 co-expression was associated with a more exhausted phenotype, with the highest PD-1 levels on TIL co-expressing Tim-3. Dampened Akt/S6 phosphorylation in these PD-1+Tim-3+ TIL, when the PD-1 pathway was ligated, suggested that signaling cross-talk could lead to escape through Tim-3 expression. Indeed, PD-1 blockade of human HNSCC TIL led to further Tim-3 upregulation, supporting a circuit of compensatory signaling and potentially permitting escape from anti-PD-1 blockade in the tumor microenvironment. Also, in a murine HNC tumor model that is partially responsive to anti-PD-1 therapy, Tim-3 was upregulated in TIL from persistently growing tumors. Significant antitumor activity was observed after sequential addition of anti-Tim-3 mAb to overcome adaptive resistance to anti-PD-1 mAb. This increased Tim-3-mediated escape of exhausted TIL from PD-1 inhibition that was mediated by phospho-inositol-3 kinase (PI3K)/Akt complex downstream of TCR signaling but not cytokine-mediated pathways. Taken together, we conclude that during PD-1 blockade, TIL upregulate Tim-3 in a PI3K/Akt-dependent manner, providing further support for dual targeting of these molecules for more effective cancer immunotherapy.
Keywords: Head and neck cancer; PD-1; Tim-3; immunotherapy; monoclonal antibody.
Figures


References
-
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492-9; PMID:21739672; http://dx.doi.org/10.1038/ni.2035 - DOI - PubMed
-
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29-37; PMID:19043418; http://dx.doi.org/10.1038/ni.1679 - DOI - PMC - PubMed
-
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670-84; PMID:17950003; http://dx.doi.org/10.1016/j.immuni.2007.09.006 - DOI - PubMed
-
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239 - DOI - PMC - PubMed
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
