Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies
- PMID: 28197421
- PMCID: PMC5289440
- DOI: 10.1038/mtm.2016.68
Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies
Abstract
Attempts to elicit antibodies with potent neutralizing activity against a broad range of human immunodeficiency virus (HIV) isolates have so far proven unsuccessful. Long-term delivery of monoclonal antibodies (mAbs) with such activity is a creative alternative that circumvents the need for an immune response and has the potential for creating a long-lasting sterilizing barrier against HIV. This approach is made possible by an incredible array of potent broadly neutralizing antibodies (bnAbs) that have been identified over the last several years. Recombinant adeno-associated virus (rAAV) vectors are ideally suited for long-term delivery for a variety of reasons. The only products made from rAAV are derived from the transgenes that are put into it; as long as those products are not viewed as foreign, expression from muscle tissue may continue for decades. Thus, use of rAAV to achieve long-term delivery of anti-HIV mAbs with potent neutralizing activity against a broad range of HIV-1 isolates is emerging as a promising concept for the prevention or treatment of HIV-1 infection in humans. Experiments in mice and monkeys that have demonstrated protective efficacy against AIDS virus infection have raised hopes for the promise of this approach. However, all published experiments in monkeys have encountered unwanted immune responses to the AAV-delivered antibody, and these immune responses appear to limit the levels of delivered antibody that can be achieved. In this review, we highlight the promise of rAAV-mediated antibody delivery for the prevention or treatment of HIV infection in humans, but we also discuss the obstacles that will need to be understood and solved in order for the promise of this approach to be realized.
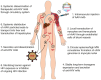
Figures



References
-
- Gottlieb, MS, Schroff, R, Schanker, HM, Weisman, JD, Fan, PT, Wolf, RA et al. (1981). Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 305: 1425–1431. - PubMed
-
- Barré-Sinoussi, F, Chermann, JC, Rey, F, Nugeyre, MT, Chamaret, S, Gruest, J et al. (1983). Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220: 868–871. - PubMed
-
- UNAIDS (2000). REPORT on the global HIV/AIDS epidemic. http://data.unaids.org/pub/Report/2000/2000_gr_en.pdf.
-
- UNAIDS (2013). Global report: UNAIDS report on the global AIDS epidemic. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Repo....
-
- Barré-Sinoussi, F, Ross, AL and Delfraissy, JF (2013). Past, present and future: 30 years of HIV research. Nat Rev Microbiol 11: 877–883. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

