Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post hoc analysis of the randomised, controlled PREVA study
- PMID: 28197844
- PMCID: PMC5309191
- DOI: 10.1186/s10194-017-0731-4
Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post hoc analysis of the randomised, controlled PREVA study
Abstract
Background: In the PREVention and Acute treatment of chronic cluster headache (PREVA) study, attack frequency reductions from baseline were significantly more pronounced with non-invasive vagus nerve stimulation plus standard of care (nVNS + SoC) than with SoC alone. Given the intensely painful and frequent nature of chronic cluster headache attacks, additional patient-centric outcomes, including the time to and level of therapeutic response, were evaluated in a post hoc analysis of the PREVA study.
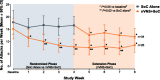
Findings: After a 2-week baseline phase, 97 patients with chronic cluster headache entered a 4-week randomised phase to receive nVNS + SoC (n = 48) or SoC alone (n = 49). All 92 patients who continued into a 4-week extension phase received nVNS + SoC. Compared with SoC alone, nVNS + SoC led to a significantly lower mean weekly attack frequency by week 2 of the randomised phase; the attack frequency remained significantly lower in the nVNS + SoC group through week 3 of the extension phase (P < 0.02). Attack frequencies in the nVNS + SoC group were significantly lower at all study time points than they were at baseline (P < 0.05). Response rates were significantly greater with nVNS + SoC than with SoC alone when response was defined as attack frequency reductions of ≥25%, ≥50%, and ≥75% from baseline (≥25% and ≥50%, P < 0.001; ≥75%, P = 0.009). The 100% response rate was 8% with nVNS + SoC and 0% with SoC alone.
Conclusions: Prophylactic nVNS led to rapid, significant, and sustained reductions in chronic cluster headache attack frequency within 2 weeks after its addition to SoC and was associated with significantly higher ≥25%, ≥50%, and ≥75% response rates than SoC alone. The rapid decrease in weekly attack frequency justifies a 4-week trial period to identify responders to nVNS, with a high degree of confidence, among patients with chronic cluster headache.
Keywords: Attack frequency; Chronic cluster headache; Non-invasive vagus nerve stimulation; PREVA; Patient-centric outcomes; Prophylactic treatment; Prophylaxis; Response rate.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

