Stimuli-responsive liposomes for drug delivery
- PMID: 28198148
- PMCID: PMC5557698
- DOI: 10.1002/wnan.1450
Stimuli-responsive liposomes for drug delivery
Abstract
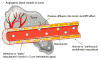
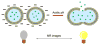
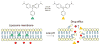
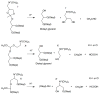
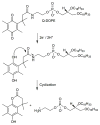
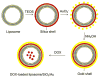
The ultimate goal of drug delivery is to increase the bioavailability and reduce the toxic side effects of the active pharmaceutical ingredient (API) by releasing them at a specific site of action. In the case of antitumor therapy, association of the therapeutic agent with a carrier system can minimize damage to healthy, nontarget tissues, while limit systemic release and promoting long circulation to enhance uptake at the cancerous site due to the enhanced permeation and retention effect (EPR). Stimuli-responsive systems have become a promising way to deliver and release payloads in a site-selective manner. Potential carrier systems have been derived from a wide variety of materials, including inorganic nanoparticles, lipids, and polymers that have been imbued with stimuli-sensitive properties to accomplish triggered release based on an environmental cue. The unique features in the tumor microenvironment can serve as an endogenous stimulus (pH, redox potential, or unique enzymatic activity) or the locus of an applied external stimulus (heat or light) to trigger the controlled release of API. In liposomal carrier systems triggered release is generally based on the principle of membrane destabilization from local defects within bilayer membranes to effect release of liposome-entrapped drugs. This review focuses on the literature appearing between November 2008-February 2016 that reports new developments in stimuli-sensitive liposomal drug delivery strategies using pH change, enzyme transformation, redox reactions, and photochemical mechanisms of activation. WIREs Nanomed Nanobiotechnol 2017, 9:e1450. doi: 10.1002/wnan.1450 For further resources related to this article, please visit the WIREs website.
© 2017 Wiley Periodicals, Inc.
Figures
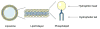




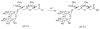
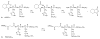
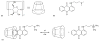


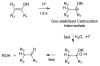
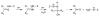

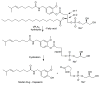







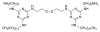




References
-
- Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J Controlled Release. 2012;160:117–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

