Interleukin-22 drives nitric oxide-dependent DNA damage and dysplasia in a murine model of colitis-associated cancer
- PMID: 28198364
- PMCID: PMC5557711
- DOI: 10.1038/mi.2017.9
Interleukin-22 drives nitric oxide-dependent DNA damage and dysplasia in a murine model of colitis-associated cancer
Abstract
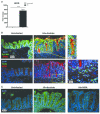
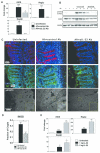
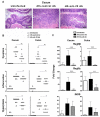
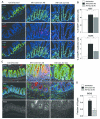
The risk of colon cancer is increased in patients with Crohn's disease and ulcerative colitis. Inflammation-induced DNA damage could be an important link between inflammation and cancer, although the pathways that link inflammation and DNA damage are incompletely defined. RAG2-deficient mice infected with Helicobacter hepaticus (Hh) develop colitis that progresses to lower bowel cancer. This process depends on nitric oxide (NO), a molecule with known mutagenic potential. We have previously hypothesized that production of NO by macrophages could be essential for Hh-driven carcinogenesis, however, whether Hh infection induces DNA damage in this model and whether this depends on NO has not been determined. Here we demonstrate that Hh infection of RAG2-deficient mice rapidly induces expression of iNOS and the development of DNA double-stranded breaks (DSBs) specifically in proliferating crypt epithelial cells. Generation of DSBs depended on iNOS activity, and further, induction of iNOS, the generation of DSBs, and the subsequent development of dysplasia were inhibited by depletion of the Hh-induced cytokine IL-22. These results demonstrate a strong association between Hh-induced DNA damage and the development of dysplasia, and further suggest that IL-22-dependent induction of iNOS within crypt epithelial cells rather than macrophages is a driving force in this process.
Figures





Similar articles
-
Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice.Proc Natl Acad Sci U S A. 2009 Jan 27;106(4):1027-32. doi: 10.1073/pnas.0812347106. Epub 2009 Jan 21. Proc Natl Acad Sci U S A. 2009. PMID: 19164562 Free PMC article.
-
Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer.Proc Natl Acad Sci U S A. 2012 Jul 3;109(27):E1820-9. doi: 10.1073/pnas.1207829109. Epub 2012 Jun 11. Proc Natl Acad Sci U S A. 2012. PMID: 22689960 Free PMC article.
-
Mutagenicity of Helicobacter hepaticus infection in the lower bowel mucosa of 129/SvEv Rag2-/- Il10-/- gpt delta mice is influenced by sex.Int J Cancer. 2019 Aug 15;145(4):1042-1054. doi: 10.1002/ijc.32332. Epub 2019 May 7. Int J Cancer. 2019. PMID: 30977112 Free PMC article.
-
Effector and regulatory CD4+ T cell function in a murine model of Helicobacter hepaticus-induced colitis.J Pediatr Gastroenterol Nutr. 2005 Apr;40 Suppl 1:S35-6. doi: 10.1097/00005176-200504001-00021. J Pediatr Gastroenterol Nutr. 2005. PMID: 15805844 Review. No abstract available.
-
Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease.Environ Mol Mutagen. 2004;44(1):3-9. doi: 10.1002/em.20024. Environ Mol Mutagen. 2004. PMID: 15199542 Review.
Cited by
-
Loss of adenomatous polyposis coli function renders intestinal epithelial cells resistant to the cytokine IL-22.PLoS Biol. 2019 Nov 26;17(11):e3000540. doi: 10.1371/journal.pbio.3000540. eCollection 2019 Nov. PLoS Biol. 2019. PMID: 31770366 Free PMC article.
-
TH9, TH17, and TH22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer.Front Oncol. 2019 Oct 4;9:1002. doi: 10.3389/fonc.2019.01002. eCollection 2019. Front Oncol. 2019. PMID: 31637216 Free PMC article. Review.
-
How autophagy, a potential therapeutic target, regulates intestinal inflammation.Front Immunol. 2023 Apr 24;14:1087677. doi: 10.3389/fimmu.2023.1087677. eCollection 2023. Front Immunol. 2023. PMID: 37168865 Free PMC article. Review.
-
Inflammatory Bowel Disease and Risk of Colorectal Cancer: An Overview From Pathophysiology to Pharmacological Prevention.Front Pharmacol. 2021 Oct 20;12:772101. doi: 10.3389/fphar.2021.772101. eCollection 2021. Front Pharmacol. 2021. PMID: 34744751 Free PMC article. Review.
-
Inflammatory bowel disease and carcinogenesis.Cancer Metastasis Rev. 2022 Jun;41(2):301-316. doi: 10.1007/s10555-022-10028-4. Epub 2022 Apr 13. Cancer Metastasis Rev. 2022. PMID: 35416564 Review.
References
-
- Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–1429. - PubMed
-
- Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23(8):1097–1104. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

