Evaluation of the immunological profile of antibody-functionalized metal-filled single-walled carbon nanocapsules for targeted radiotherapy
- PMID: 28198410
- PMCID: PMC5309841
- DOI: 10.1038/srep42605
Evaluation of the immunological profile of antibody-functionalized metal-filled single-walled carbon nanocapsules for targeted radiotherapy
Abstract
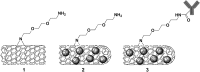
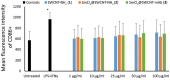
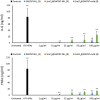
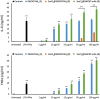
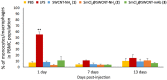
This study investigates the immune responses induced by metal-filled single-walled carbon nanotubes (SWCNT) under in vitro, ex vivo and in vivo settings. Either empty amino-functionalized CNTs [SWCNT-NH2 (1)] or samarium chloride-filled amino-functionalized CNTs with [SmCl3@SWCNT-mAb (3)] or without [SmCl3@SWCNT-NH2 (2)] Cetuximab functionalization were tested. Conjugates were added to RAW 264.7 or PBMC cells in a range of 1 μg/ml to 100 μg/ml for 24 h. Cell viability and IL-6/TNFα production were determined by flow cytometry and ELISA. Additionally, the effect of SWCNTs on the number of T lymphocytes, B lymphocytes and monocytes within the PBMC subpopulations was evaluated by immunostaining and flow cytometry. The effect on monocyte number in living mice was assessed after tail vein injection (150 μg of each conjugate per mouse) at 1, 7 and 13 days post-injection. Overall, our study showed that all the conjugates had no significant effect on cell viability of RAW 264.7 but conjugates 1 and 3 led to a slight increase in IL-6/TNFα. All the conjugates resulted in significant reduction in monocyte/macrophage cell numbers within PBMCs in a dose-dependent manner. Interestingly, monocyte depletion was not observed in vivo, suggesting their suitability for future testing in the field of targeted radiotherapy in mice.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Functionalization of filled radioactive multi-walled carbon nanocapsules by arylation reaction for in vivo delivery of radio-therapy.J Mater Chem B. 2021 Dec 22;10(1):47-56. doi: 10.1039/d1tb02195h. J Mater Chem B. 2021. PMID: 34843615
-
Protective role of functionalized single walled carbon nanotubes enhance ex vivo expansion of hematopoietic stem and progenitor cells in human umbilical cord blood.Nanomedicine. 2013 Nov;9(8):1304-16. doi: 10.1016/j.nano.2013.05.009. Epub 2013 Jun 1. Nanomedicine. 2013. PMID: 23732300
-
Design of antibody-functionalized carbon nanotubes filled with radioactivable metals towards a targeted anticancer therapy.Nanoscale. 2016 Jul 7;8(25):12626-38. doi: 10.1039/c5nr07923c. Epub 2016 Jan 6. Nanoscale. 2016. PMID: 26733445
-
Poly-dispersed Acid-Functionalized Single-Walled Carbon Nanotubes (AF-SWCNTs) Are Potent Inhibitor of BCG Induced Inflammatory Response in Macrophages.Inflammation. 2021 Jun;44(3):908-922. doi: 10.1007/s10753-020-01386-8. Epub 2021 Jan 5. Inflammation. 2021. PMID: 33400104
-
Enhanced antibody response to ovalbumin coupled to poly-dispersed acid functionalized single walled carbon nanotubes.Immunol Lett. 2020 Jan;217:77-83. doi: 10.1016/j.imlet.2019.11.003. Epub 2019 Nov 11. Immunol Lett. 2020. PMID: 31726189
Cited by
-
Nanoparticle Effects on Stress Response Pathways and Nanoparticle-Protein Interactions.Int J Mol Sci. 2022 Jul 19;23(14):7962. doi: 10.3390/ijms23147962. Int J Mol Sci. 2022. PMID: 35887304 Free PMC article. Review.
-
Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine.Nanomaterials (Basel). 2021 Nov 10;11(11):3020. doi: 10.3390/nano11113020. Nanomaterials (Basel). 2021. PMID: 34835783 Free PMC article. Review.
-
Applications of Filled Single-Walled Carbon Nanotubes: Progress, Challenges, and Perspectives.Nanomaterials (Basel). 2021 Oct 27;11(11):2863. doi: 10.3390/nano11112863. Nanomaterials (Basel). 2021. PMID: 34835628 Free PMC article. Review.
-
Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots.Nanomaterials (Basel). 2023 Apr 25;13(9):1458. doi: 10.3390/nano13091458. Nanomaterials (Basel). 2023. PMID: 37177003 Free PMC article. Review.
-
Metal Nanocomposites as Biosensors for Biological Fluids Analysis.Materials (Basel). 2025 Apr 15;18(8):1809. doi: 10.3390/ma18081809. Materials (Basel). 2025. PMID: 40333451 Free PMC article. Review.
References
-
- Bianco A., Kostarelos K., Partidos C. D. & Prato M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 571 (2005). - PubMed
-
- Hong G., Diao S., Antaris A. L. & Dai H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 115, 10816–10906 (2015). - PubMed
-
- Ménard-Moyon C., Kostarelos K., Prato M. & Bianco A. Functionalized carbon nanotubes for probing and modulating molecular functions. Chem. Biol. 17, 107–115 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

