Human IDO-competent, long-lived immunoregulatory dendritic cells induced by intracellular pathogen, and their fate in humanized mice
- PMID: 28198424
- PMCID: PMC5309771
- DOI: 10.1038/srep41083
Human IDO-competent, long-lived immunoregulatory dendritic cells induced by intracellular pathogen, and their fate in humanized mice
Abstract
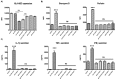
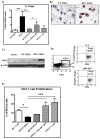
Targeting of myeloid-dendritic cell receptor DC-SIGN by numerous chronic infectious agents, including Porphyromonas gingivalis, is shown to drive-differentiation of monocytes into dysfunctional mDCs. These mDCs exhibit alterations of their fine-tuned homeostatic function and contribute to dysregulated immune-responses. Here, we utilize P. gingivalis mutant strains to show that pathogen-differentiated mDCs from primary human-monocytes display anti-apoptotic profile, exhibited by elevated phosphorylated-Foxo1, phosphorylated-Akt1, and decreased Bim-expression. This results in an overall inhibition of DC-apoptosis. Direct stimulation of complex component CD40 on DCs leads to activation of Akt1, suggesting CD40 involvement in anti-apoptotic effects observed. Further, these DCs drove dampened CD8+ T-cell and Th1/Th17 effector-responses while inducing CD25+Foxp3+CD127- Tregs. In vitro Treg induction was mediated by DC expression of indoleamine 2,3-dioxygenase, and was confirmed in IDO-KO mouse model. Pathogen-infected &CMFDA-labeled MoDCs long-lasting survival was confirmed in a huMoDC reconstituted humanized mice. In conclusion, our data implicate PDDCs as an important target for resolution of chronic infection.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Dejnirattisai W. et al.. A complex interplay among virus, dendritic cells, T cells, and cytokines in dengue virus infections. Journal of immunology (Baltimore, Md.: 1950) 181, 5865–5874 (2008). - PubMed
-
- van Kooten C. et al.. Dendritic cells as a tool to induce transplantation tolerance: obstacles and opportunities. Transplantation 91, 2–7 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

