Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization
- PMID: 28199033
- PMCID: PMC5469720
- DOI: 10.1002/adma.201606209
Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization
Abstract
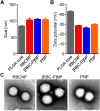
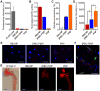
Cell-membrane-coated nanoparticles have recently been studied extensively for their biological compatibility, retention of cellular properties, and adaptability to a variety of therapeutic and imaging applications. This class of nanoparticles, which has been fabricated with a variety of cell membrane coatings, including those derived from red blood cells (RBCs), platelets, white blood cells, cancer cells, and bacteria, exhibit properties that are characteristic of the source cell. In this study, a new type of biological coating is created by fusing membrane material from two different cells, providing a facile method for further enhancing nanoparticle functionality. As a proof of concept, the development of dual-membrane-coated nanoparticles from the fused RBC membrane and platelet membrane is demonstrated. The resulting particles, termed RBC-platelet hybrid membrane-coated nanoparticles ([RBC-P]NPs), are thoroughly characterized, and it is shown that they carry properties of both source cells. Further, the [RBC-P]NP platform exhibits long circulation and suitability for further in vivo exploration. The reported strategy opens the door for the creation of biocompatible, custom-tailored biomimetic nanoparticles with varying hybrid functionalities, which may be used to overcome the limitations of current nanoparticle-based therapeutic and imaging platforms.
Keywords: biomimetic nanoparticles; long circulation; membrane fusion; nanomedicine; targeted delivery.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



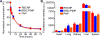
References
-
- Hu CMJ, Fang RH, Luk BT, Zhang L. Nanoscale. 2014;6:65. - PubMed
-
- Farokhzad OC, Langer R. ACS Nano. 2009;3:16. - PubMed
-
- Allen TM, Cullis PR. Adv Drug Deliv Rev. 2013;65:36. - PubMed
-
- Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Nanomedicine. 2007;2:23. - PubMed
-
- Brannon-Peppas L, Blanchette JO. Adv Drug Deliv Rev. 2004;56:1649. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

