Cobalt-Catalyzed 1,1-Diboration of Terminal Alkynes: Scope, Mechanism, and Synthetic Applications
- PMID: 28199104
- PMCID: PMC5697746
- DOI: 10.1021/jacs.7b00445
Cobalt-Catalyzed 1,1-Diboration of Terminal Alkynes: Scope, Mechanism, and Synthetic Applications
Abstract
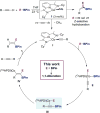
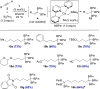
A cobalt-catalyzed method for the 1,1-diboration of terminal alkynes with bis(pinacolato)diboron (B2Pin2) is described. The reaction proceeds efficiently at 23 °C with excellent 1,1-selectivity and broad functional group tolerance. With the unsymmetrical diboron reagent PinB-BDan (Dan = naphthalene-1,8-diaminato), stereoselective 1,1-diboration provided products with two boron substituents that exhibit differential reactivity. One example prepared by diboration of 1-octyne was crystallized, and its stereochemistry established by X-ray crystallography. The utility and versatility of the 1,1-diborylalkene products was demonstrated in a number of synthetic applications, including a concise synthesis of the epilepsy medication tiagabine. In addition, a synthesis of 1,1,1-triborylalkanes was accomplished through cobalt-catalyzed hydroboration of 1,1-diborylalkenes with HBPin. Deuterium-labeling and stoichiometric experiments support a mechanism involving selective insertion of an alkynylboronate to a Co-B bond of a cobalt boryl complex to form a vinylcobalt intermediate. The latter was isolated and characterized by NMR spectroscopy and X-ray crystallography. A competition experiment established that the reaction involves formation of free alkynylboronate and the two boryl substituents are not necessarily derived from the same diboron source.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
- Fernández E, Whiting A, editors. Synthesis and Application of Organoboron Compounds. Springer; Berlin: 2015.
- Hall DG, editor. Boronic Acids. Wiley-VCH; Weinheim: 2011.
- Miyaura N, Suzuki A. Chem Rev. 1995;95:2457.
- Miyaura N. Bull Chem Soc Jpn. 2008;81:1535.
-
-
For selected key examples, see:
- Ishiyama T, Yamamoto M, Miyaura N. Chem Lett. 1996;25:1117.
- Brown SD, Armstrong RW. J Am Chem Soc. 1996;118:6331.
- Brown SD, Armstrong RW. J Org Chem. 1997;62:7076. - PubMed
- Iwadate N, Suginome M. J Am Chem Soc. 2010;132:2548. - PubMed
-
For a review, see:
- Iwasaki M, Nishihara Y. Chem Rec. 2016;16:2031. - PubMed
-
-
- Wang J, editor. Stereoselective Alkene Synthesis in Topics in Current Chemistry. Springer; Berlin: 2012.
- Negishi E, Huang Z, Wang G, Mohan S, Wang C, Hattori H. Acc Chem Res. 2008;41:1474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

