Sterile protection against human malaria by chemoattenuated PfSPZ vaccine
- PMID: 28199305
- PMCID: PMC10906480
- DOI: 10.1038/nature21060
Sterile protection against human malaria by chemoattenuated PfSPZ vaccine
Abstract
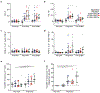
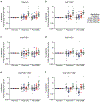
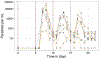
A highly protective malaria vaccine would greatly facilitate the prevention and elimination of malaria and containment of drug-resistant parasites. A high level (more than 90%) of protection against malaria in humans has previously been achieved only by immunization with radiation-attenuated Plasmodium falciparum (Pf) sporozoites (PfSPZ) inoculated by mosquitoes; by intravenous injection of aseptic, purified, radiation-attenuated, cryopreserved PfSPZ ('PfSPZ Vaccine'); or by infectious PfSPZ inoculated by mosquitoes to volunteers taking chloroquine or mefloquine (chemoprophylaxis with sporozoites). We assessed immunization by direct venous inoculation of aseptic, purified, cryopreserved, non-irradiated PfSPZ ('PfSPZ Challenge') to malaria-naive, healthy adult volunteers taking chloroquine for antimalarial chemoprophylaxis (vaccine approach denoted as PfSPZ-CVac). Three doses of 5.12 × 104 PfSPZ of PfSPZ Challenge at 28-day intervals were well tolerated and safe, and prevented infection in 9 out of 9 (100%) volunteers who underwent controlled human malaria infection ten weeks after the last dose (group III). Protective efficacy was dependent on dose and regimen. Immunization with 3.2 × 103 (group I) or 1.28 × 104 (group II) PfSPZ protected 3 out of 9 (33%) or 6 out of 9 (67%) volunteers, respectively. Three doses of 5.12 × 104 PfSPZ at five-day intervals protected 5 out of 8 (63%) volunteers. The frequency of Pf-specific polyfunctional CD4 memory T cells was associated with protection. On a 7,455 peptide Pf proteome array, immune sera from at least 5 out of 9 group III vaccinees recognized each of 22 proteins. PfSPZ-CVac is a highly efficacious vaccine candidate; when we are able to optimize the immunization regimen (dose, interval between doses, and drug partner), this vaccine could be used for combination mass drug administration and a mass vaccination program approach to eliminate malaria from geographically defined areas.
Conflict of interest statement
The authors declare competing financial interests: details are available in the
Figures
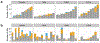







References
-
- Clyde DF, Most H, McCarthy VC & Vanderberg JP Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci 266, 169–177 (1973). - PubMed
-
- Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS & Sell KW Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg 68, 258–259 (1974). - PubMed
-
- Hoffman SL et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis 185, 1155–1164 (2002). - PubMed
-
- Seder RA et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

