Implementation of fluorescence confocal mosaicking microscopy by "early adopter" Mohs surgeons and dermatologists: recent progress
- PMID: 28199474
- PMCID: PMC5310648
- DOI: 10.1117/1.JBO.22.2.024002
Implementation of fluorescence confocal mosaicking microscopy by "early adopter" Mohs surgeons and dermatologists: recent progress
Abstract
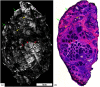
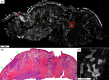
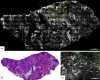
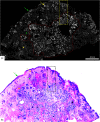
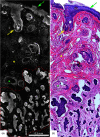
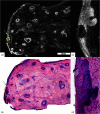
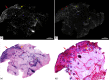
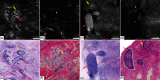
Confocal mosaicking microscopy (CMM) enables rapid imaging of large areas of fresh tissue ex vivo without the processing that is necessary for conventional histology. When performed in fluorescence mode using acridine orange (nuclear specific dye), it enhances nuclei-to-dermis contrast that enables detection of all types of basal cell carcinomas (BCCs), including micronodular and thin strands of infiltrative types. So far, this technique has been mostly validated in research settings for the detection of residual BCC tumor margins with high sensitivity of 89% to 96% and specificity of 99% to 89%. Recently, CMM has advanced to implementation and testing in clinical settings by “early adopter” Mohs surgeons, as an adjunct to frozen section during Mohs surgery. We summarize the development of CMM guided imaging of ex vivo skin tissues from bench to bedside. We also present its current state of application in routine clinical workflow not only for the assessment of residual BCC margins in the Mohs surgical setting but also for some melanocytic lesions and other skin conditions in clinical dermatology settings. Last, we also discuss the potential limitations of this technology as well as future developments. As this technology advances further, it may serve as an adjunct to standard histology and enable rapid surgical pathology of skin cancers at the bedside.
Figures

Similar articles
-
Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy.Br J Dermatol. 2009 Jun;160(6):1242-50. doi: 10.1111/j.1365-2133.2009.09141.x. Epub 2009 Mar 30. Br J Dermatol. 2009. PMID: 19416248 Free PMC article.
-
Basal cell carcinoma characterization using fusion ex vivo confocal microscopy: a promising change in conventional skin histopathology.Br J Dermatol. 2020 Feb;182(2):468-476. doi: 10.1111/bjd.18239. Epub 2019 Oct 27. Br J Dermatol. 2020. PMID: 31220341 Free PMC article.
-
Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery.Br J Dermatol. 2014 Feb;170(2):360-5. doi: 10.1111/bjd.12671. Br J Dermatol. 2014. PMID: 24117457
-
In Vivo and Ex Vivo Confocal Microscopy for Dermatologic and Mohs Surgeons.Dermatol Clin. 2016 Oct;34(4):497-504. doi: 10.1016/j.det.2016.05.012. Dermatol Clin. 2016. PMID: 27692455 Free PMC article. Review.
-
Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions.Acta Dermatovenerol Croat. 2018 Apr;26(1):64-67. Acta Dermatovenerol Croat. 2018. PMID: 29782304 Review.
Cited by
-
Molecular imaging and validation of margins in surgically excised nonmelanoma skin cancer specimens.J Med Imaging (Bellingham). 2019 Jan;6(1):016001. doi: 10.1117/1.JMI.6.1.016001. Epub 2019 Mar 18. J Med Imaging (Bellingham). 2019. PMID: 30915384 Free PMC article.
-
Quantitative and comparative assessment of dyes and protocols for rapid ex vivo microscopy of fresh tissues.Sci Rep. 2024 Sep 13;14(1):21376. doi: 10.1038/s41598-024-72213-5. Sci Rep. 2024. PMID: 39271788 Free PMC article.
-
Acridine Orange: A Review of Novel Applications for Surgical Cancer Imaging and Therapy.Front Oncol. 2019 Sep 24;9:925. doi: 10.3389/fonc.2019.00925. eCollection 2019. Front Oncol. 2019. PMID: 31612102 Free PMC article. Review.
-
Piston-based specimen holder for rapid surgical and biopsy specimen imaging.Biomed Opt Express. 2024 Apr 8;15(5):2898-2909. doi: 10.1364/BOE.522379. eCollection 2024 May 1. Biomed Opt Express. 2024. PMID: 38855659 Free PMC article.
-
Exploring the utility of Deep Red Anthraquinone 5 for digital staining of ex vivo confocal micrographs of optically sectioned skin.J Biophotonics. 2021 Apr;14(4):e202000207. doi: 10.1002/jbio.202000207. Epub 2020 Dec 28. J Biophotonics. 2021. PMID: 33314673 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

