Higher T-Cell Responses Induced by DNA/rAd5 HIV-1 Preventive Vaccine Are Associated With Lower HIV-1 Infection Risk in an Efficacy Trial
- PMID: 28199679
- PMCID: PMC5853653
- DOI: 10.1093/infdis/jix086
Higher T-Cell Responses Induced by DNA/rAd5 HIV-1 Preventive Vaccine Are Associated With Lower HIV-1 Infection Risk in an Efficacy Trial
Abstract
Background: It is important to identify vaccine-induced immune responses that predict the preventative efficacy of a human immunodeficiency virus (HIV)-1 vaccine. We assessed T-cell response markers as correlates of risk in the HIV Vaccine Trials Network (HVTN) 505 HIV-1 vaccine efficacy trial.
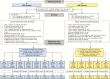
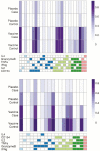
Methods: 2504 participants were randomized to DNA/rAd5 vaccine or placebo, administered at weeks 0, 4, 8, and 24. Peripheral blood mononuclear cells were obtained at week 26 from all 25 primary endpoint vaccine cases and 125 matched vaccine controls, and stimulated with vaccine-insert-matched peptides. Primary variables were total HIV-1-specific CD4+ T-cell magnitude and Env-specific CD4+ polyfunctionality. Four secondary variables were also assessed. Immune responses were evaluated as predictors of HIV-1 infection among vaccinees using Cox proportional hazards models. Machine learning analyses identified immune response combinations best predicting HIV-1 infection.
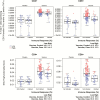
Results: We observed an unexpectedly strong inverse correlation between Env-specific CD8+ immune response magnitude and HIV-1 infection risk (hazard ratio [HR] = 0.18 per SD increment; P = .04) and between Env-specific CD8+ polyfunctionality and infection risk (HR = 0.34 per SD increment; P < .01).
Conclusions: Further research is needed to determine if these immune responses are predictors of vaccine efficacy or markers of natural resistance to HIV-1 infection.
Keywords: HIV-1 vaccine; HVTN 505 vaccine efficacy trial; T-cell immunogenicity; T-cell polyfunctionality; correlates of risk; intracellular cytokine staining; machine learning analyses; vaccine-induced immune response..
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF; rgp120 HIV Vaccine Study Group Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191:654–65. - PubMed
-
- Pitisuttithum P, Gilbert P, Gurwith M, et al. ; Bangkok Vaccine Evaluation Group. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006; 194:1661–71. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. ; MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

