Human CTF18-RFC clamp-loader complexed with non-synthesising DNA polymerase ε efficiently loads the PCNA sliding clamp
- PMID: 28199690
- PMCID: PMC5416766
- DOI: 10.1093/nar/gkx096
Human CTF18-RFC clamp-loader complexed with non-synthesising DNA polymerase ε efficiently loads the PCNA sliding clamp
Abstract
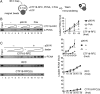
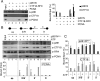
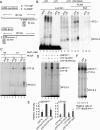
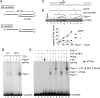
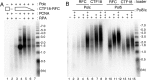
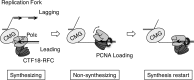
The alternative proliferating-cell nuclear antigen (PCNA)-loader CTF18-RFC forms a stable complex with DNA polymerase ε (Polε). We observed that, under near-physiological conditions, CTF18-RFC alone loaded PCNA inefficiently, but loaded it efficiently when complexed with Polε. During efficient PCNA loading, CTF18-RFC and Polε assembled at a 3΄ primer-template junction cooperatively, and directed PCNA to the loading site. Site-specific photo-crosslinking of directly interacting proteins at the primer-template junction showed similar cooperative binding, in which the catalytic N-terminal portion of Polε acted as the major docking protein. In the PCNA-loading intermediate with ATPγS, binding of CTF18 to the DNA structures increased, suggesting transient access of CTF18-RFC to the primer terminus. Polε placed in DNA synthesis mode using a substrate DNA with a deoxidised 3΄ primer end did not stimulate PCNA loading, suggesting that DNA synthesis and PCNA loading are mutually exclusive at the 3΄ primer-template junction. Furthermore, PCNA and CTF18-RFC-Polε complex engaged in stable trimeric assembly on the template DNA and synthesised DNA efficiently. Thus, CTF18-RFC appears to be involved in leading-strand DNA synthesis through its interaction with Polε, and can load PCNA onto DNA when Polε is not in DNA synthesis mode to restore DNA synthesis.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

