Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
- PMID: 28199818
- PMCID: PMC5455807
- DOI: 10.1200/JCO.2016.70.7398
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
Erratum in
-
Errata.J Clin Oncol. 2017 Nov 10;35(32):3736. doi: 10.1200/JCO.2017.76.3292. J Clin Oncol. 2017. PMID: 29112824 Free PMC article. No abstract available.
-
Errata.J Clin Oncol. 2018 Feb 10;36(5):521. doi: 10.1200/JCO.2017.77.6526. J Clin Oncol. 2018. PMID: 29414281 Free PMC article. No abstract available.
Abstract
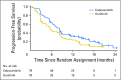
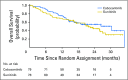
Purpose Cabozantinib is an oral potent inhibitor of vascular endothelial growth factor receptor 2, MET, and AXL and is a standard second-line therapy for metastatic renal cell carcinoma (mRCC). This randomized phase II multicenter trial evaluated cabozantinib compared with sunitinib as first-line therapy in patients with mRCC. Patients and Methods Eligible patients had untreated clear cell mRCC and Eastern Cooperative Oncology Group performance status of 0 to 2 and were intermediate or poor risk per International Metastatic Renal Cell Carcinoma Database Consortium criteria. Patients were randomly assigned at a one-to-one ratio to cabozantinib (60 mg once per day) or sunitinib (50 mg once per day; 4 weeks on, 2 weeks off). Progression-free survival (PFS) was the primary end point. Objective response rate (ORR), overall survival, and safety were secondary end points. Results From July 2013 to April 2015, 157 patients were randomly assigned (cabozantinib, n = 79; sunitinib, n = 78). Compared with sunitinib, cabozantinib treatment significantly increased median PFS (8.2 v 5.6 months) and was associated with a 34% reduction in rate of progression or death (adjusted hazard ratio, 0.66; 95% CI, 0.46 to 0.95; one-sided P = .012). ORR was 33% (95% CI, 23 to 44) for cabozantinib versus 12% (95% CI, 5.4 to 21) for sunitinib. All-causality grade 3 or 4 adverse events were 67% for cabozantinib and 68% for sunitinib and included diarrhea (cabozantinib, 10% v sunitinib, 11%), fatigue (6% v 15%), hypertension (28% v 22%), palmar-plantar erythrodysesthesia (8% v 4%), and hematologic adverse events (3% v 22%). Conclusion Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
Figures


Comment in
-
Future Challenges for Drug Development in Renal Cell Carcinoma.J Clin Oncol. 2017 Feb 20;35(6):577-579. doi: 10.1200/JCO.2016.71.0673. Epub 2016 Dec 19. J Clin Oncol. 2017. PMID: 27992269 No abstract available.
-
Reply to B. Rini et al and S. Buti et al.J Clin Oncol. 2017 Jun 1;35(16):1859-1860. doi: 10.1200/JCO.2017.72.2629. Epub 2017 Mar 6. J Clin Oncol. 2017. PMID: 28549224 No abstract available.
-
Is Cabozantinib Really Better Than Sunitinib As First-Line Treatment of Metastatic Renal Cell Carcinoma?J Clin Oncol. 2017 Jun 1;35(16):1858-1859. doi: 10.1200/JCO.2016.71.6506. Epub 2017 Mar 6. J Clin Oncol. 2017. PMID: 28549228 No abstract available.
References
-
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. - PubMed
-
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. - PubMed
-
- Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2015. J Natl Compr Canc Netw. 2015;13:151–159. - PubMed
-
- Powles T, Staehler M, Ljungberg B, et al. Updated EAU guidelines for clear cell renal cancer patients who fail VEGF targeted therapy. Eur Urol. 2016;69:4–6. - PubMed
-
- Hackshaw MD, Holmes M, Lankford M, et al. Prescribing preferences in the first-line treatment for patients with metastatic renal cell carcinoma in the United States. Clin Genitourin Cancer. 2016;14:e479–e487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

