Neuropilin-1 Is Expressed on Lymphoid Tissue Residing LTi-like Group 3 Innate Lymphoid Cells and Associated with Ectopic Lymphoid Aggregates
- PMID: 28199847
- PMCID: PMC5318658
- DOI: 10.1016/j.celrep.2017.01.063
Neuropilin-1 Is Expressed on Lymphoid Tissue Residing LTi-like Group 3 Innate Lymphoid Cells and Associated with Ectopic Lymphoid Aggregates
Abstract
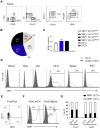
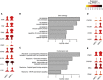
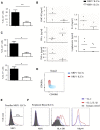
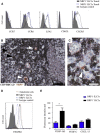
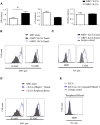
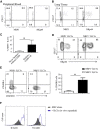
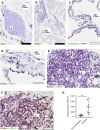
Here, we characterize a subset of ILC3s that express Neuropilin1 (NRP1) and are present in lymphoid tissues, but not in the peripheral blood or skin. NRP1+ group 3 innate lymphoid cells (ILC3s) display in vitro lymphoid tissue inducer (LTi) activity. In agreement with this, NRP1+ ILC3s are mainly located in proximity to high endothelial venules (HEVs) and express cell surface molecules involved in lymphocyte migration in secondary lymphoid tissues via HEVs. NRP1 was also expressed on mouse fetal LTi cells, indicating that NRP1 is a conserved marker for LTi cells. Human NRP1+ ILC3s are primed cells because they express CD45RO and produce higher amounts of cytokines than NRP1- cells, which express CD45RA. The NRP1 ligand vascular endothelial growth factor A (VEGF-A) served as a chemotactic factor for NRP1+ ILC3s. NRP1+ ILC3s are present in lung tissues from smokers and patients with chronic obstructive pulmonary disease, suggesting a role in angiogenesis and/or the initiation of ectopic pulmonary lymphoid aggregates.
Keywords: HEVs; ILC3; LA; LTi; NRP1; VEGF-A; chemotaxis; high endothelial venues; human innate lymphoid cells 3; lymphoid tissue inducer cells; neuropilin-1; smoke-induced pulmonary lymphoid aggregates; vascular endothelial growth factor A.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. - PubMed
-
- Bal S.M., Bernink J.H., Nagasawa M., Groot J., Shikhagaie M.M., Golebski K., van Drunen C.M., Lutter R., Jonkers R.E., Hombrink P. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17:636–645. - PubMed
-
- Bernink J.H., Krabbendam L., Germar K., de Jong E., Gronke K., Kofoed-Nielsen M., Munneke J.M., Hazenberg M.D., Villaudy J., Buskens C.J. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43:146–160. - PubMed
-
- Björklund Å.K., Forkel M., Picelli S., Konya V., Theorell J., Friberg D., Sandberg R., Mjösberg J. The heterogeneity of human CD127 innate lymphoid cells revealed by single-cell RNA sequencing. Nat. Immunol. 2016;17:451–460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

