In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen
- PMID: 28201509
- PMCID: PMC5890668
- DOI: 10.1093/jac/dkx020
In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen
Abstract
Objectives: T-2307, a novel arylamidine, exhibits potent broad-spectrum activities against the majority of fungal pathogens. In this study, the antifungal activity of T-2307 against Cryptococcus gattii was evaluated in comparison with those of amphotericin B, fluconazole and voriconazole in vitro and in vivo .
Methods: The MICs for 15 clinical isolates were determined according to CLSI guidelines and time-kill studies were performed using C. gattii YF2784. In a murine model for intranasal pulmonary infection caused by C. gattii YF2784, the test compounds were administered once daily for 7 days from 2 h or 14 days post-infection. The viable counts in the lungs and brain were determined at 21 days post-infection.
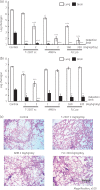
Results: The MIC range, MIC 50 , MIC 90 and geometric mean MIC of T-2307 were 0.0078-0.0625, 0.0313, 0.0625 and 0.0394 mg/L, respectively. The MIC of T-2307 was significantly lower than those of fluconazole, voriconazole and amphotericin B. T-2307 showed concentration-dependent fungicidal activity at 4 times the MIC or higher. Administration of T-2307 at 2 mg/kg/day, amphotericin B at 1 mg/kg/day and fluconazole at 160 mg/kg/day from 2 h post-infection significantly reduced viable counts in the lungs and brain. However, when the administration was started 14 days post-infection, only T-2307 significantly reduced the viable counts in both the lungs and the brain at 1 mg/kg/day.
Conclusions: T-2307 shows excellent in vitro and in vivo antifungal activities against C. gattii and would be a promising new candidate for the treatment of cryptococcosis.
© The Author 2017. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Molecular epidemiology and in vitro antifungal susceptibility testing of 108 clinical Cryptococcus neoformans sensu lato and Cryptococcus gattii sensu lato isolates from Denmark.Mycoses. 2016 Sep;59(9):576-84. doi: 10.1111/myc.12507. Epub 2016 Apr 8. Mycoses. 2016. PMID: 27061834
-
Comparative antifungal susceptibility analyses of Cryptococcus neoformans VNI and Cryptococcus gattii VGII from the Brazilian Amazon Region by the Etest, Vitek 2, and the Clinical and Laboratory Standards Institute broth microdilution methods.Med Mycol. 2019 Oct 1;57(7):864-873. doi: 10.1093/mmy/myy150. Med Mycol. 2019. PMID: 30657975
-
Correlation of the in vitro antifungal drug susceptibility with the in vivo activity of fluconazole in a murine model of cerebral infection caused by Cryptococcus gattii.Eur J Clin Microbiol Infect Dis. 2010 Dec;29(12):1525-32. doi: 10.1007/s10096-010-1034-8. Epub 2010 Aug 28. Eur J Clin Microbiol Infect Dis. 2010. PMID: 20803047
-
Cryptococcosis-a systematic review to inform the World Health Organization Fungal Priority Pathogens List.Med Mycol. 2024 Jun 27;62(6):myae043. doi: 10.1093/mmy/myae043. Med Mycol. 2024. PMID: 38935902 Free PMC article.
-
Antifungal Resistance in Cryptococcal Infections.Pathogens. 2024 Jan 29;13(2):128. doi: 10.3390/pathogens13020128. Pathogens. 2024. PMID: 38392866 Free PMC article. Review.
Cited by
-
Advances in Antifungal Drug Development: An Up-To-Date Mini Review.Pharmaceuticals (Basel). 2021 Dec 16;14(12):1312. doi: 10.3390/ph14121312. Pharmaceuticals (Basel). 2021. PMID: 34959712 Free PMC article. Review.
-
Recent Progress in Research on Mitochondrion-Targeted Antifungal Drugs: a Review.Antimicrob Agents Chemother. 2023 Jun 15;67(6):e0000323. doi: 10.1128/aac.00003-23. Epub 2023 May 17. Antimicrob Agents Chemother. 2023. PMID: 37195189 Free PMC article. Review.
-
Review of T-2307, an Investigational Agent That Causes Collapse of Fungal Mitochondrial Membrane Potential.J Fungi (Basel). 2021 Feb 11;7(2):130. doi: 10.3390/jof7020130. J Fungi (Basel). 2021. PMID: 33670132 Free PMC article. Review.
-
ATI-2307 Exhibits Equivalent Antifungal Activity in Cryptococcus neoformans Clinical Isolates With High and Low Fluconazole IC50.Front Cell Infect Microbiol. 2021 Jun 23;11:695240. doi: 10.3389/fcimb.2021.695240. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34249782 Free PMC article.
-
Pulmonary Cryptococcosis.J Fungi (Basel). 2022 Oct 31;8(11):1156. doi: 10.3390/jof8111156. J Fungi (Basel). 2022. PMID: 36354923 Free PMC article. Review.
References
-
- Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol 2001; 39: 155–68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

