Frozen in Time: The History of Proteins
- PMID: 28201543
- PMCID: PMC5400399
- DOI: 10.1093/molbev/msx086
Frozen in Time: The History of Proteins
Abstract
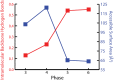
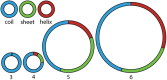
The ribosome is imprinted with a detailed molecular chronology of the origins and early evolution of proteins. Here we show that when arranged by evolutionary phase of ribosomal evolution, ribosomal protein (rProtein) segments reveal an atomic level history of protein folding. The data support a model in which aboriginal oligomers evolved into globular proteins in a hierarchical step-wise process. Complexity of assembly and folding of polypeptide increased incrementally in concert with expansion of rRNA. (i) Short random coil proto-peptides bound to rRNA, and (ii) lengthened over time and coalesced into β-β secondary elements. These secondary elements (iii) accreted and collapsed, primarily into β-domains. Domains (iv) accumulated and gained complex super-secondary structures composed of mixtures of α-helices and β-strands. Early protein evolution was guided and accelerated by interactions with rRNA. rRNA and proto-peptide provided mutual protection from chemical degradation and disassembly. rRNA stabilized polypeptide assemblies, which evolved in a stepwise process into globular domains, bypassing the immense space of random unproductive sequences. Coded proteins originated as oligomers and polymers created by the ribosome, on the ribosome and for the ribosome. Synthesis of increasingly longer products was iteratively coupled with lengthening and maturation of the ribosomal exit tunnel. Protein catalysis appears to be a late byproduct of selection for sophisticated and finely controlled assembly.
Keywords: origin of life; origins of protein folding; protein evolution; ribosomal origins and evolution; ribosomal protein; β-harpin.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures
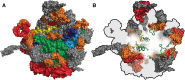


Similar articles
-
Ancestral Interactions of Ribosomal RNA and Ribosomal Proteins.Biophys J. 2017 Jul 25;113(2):268-276. doi: 10.1016/j.bpj.2017.04.007. Epub 2017 May 12. Biophys J. 2017. PMID: 28506527 Free PMC article.
-
The ribosome as a missing link in prebiotic evolution II: Ribosomes encode ribosomal proteins that bind to common regions of their own mRNAs and rRNAs.J Theor Biol. 2016 May 21;397:115-27. doi: 10.1016/j.jtbi.2016.02.030. Epub 2016 Mar 4. J Theor Biol. 2016. PMID: 26953650
-
Root of the Tree: The Significance, Evolution, and Origins of the Ribosome.Chem Rev. 2020 Jun 10;120(11):4848-4878. doi: 10.1021/acs.chemrev.9b00742. Epub 2020 May 6. Chem Rev. 2020. PMID: 32374986 Review.
-
Mechanistic Insight into the Reactivation of BCAII Enzyme from Denatured and Molten Globule States by Eukaryotic Ribosomes and Domain V rRNAs.PLoS One. 2016 Apr 21;11(4):e0153928. doi: 10.1371/journal.pone.0153928. eCollection 2016. PLoS One. 2016. PMID: 27099964 Free PMC article.
-
Ribosomal proteins: structure, function, and evolution.Biochemistry (Mosc). 2012 Jun;77(6):562-74. doi: 10.1134/S0006297912060028. Biochemistry (Mosc). 2012. PMID: 22817455 Review.
Cited by
-
The interplay between peptides and RNA is critical for protoribosome compartmentalization and stability.Nucleic Acids Res. 2024 Nov 11;52(20):12689-12700. doi: 10.1093/nar/gkae823. Nucleic Acids Res. 2024. PMID: 39340303 Free PMC article.
-
The Expanding Universe of Extensions and Tails: Ribosomal Proteins and Histones in RNA and DNA Complex Signaling and Dynamics.Genes (Basel). 2025 Jan 1;16(1):45. doi: 10.3390/genes16010045. Genes (Basel). 2025. PMID: 39858592 Free PMC article. Review.
-
TwinCons: Conservation score for uncovering deep sequence similarity and divergence.PLoS Comput Biol. 2021 Oct 29;17(10):e1009541. doi: 10.1371/journal.pcbi.1009541. eCollection 2021 Oct. PLoS Comput Biol. 2021. PMID: 34714829 Free PMC article.
-
On the Nature of the Last Common Ancestor: A Story from its Translation Machinery.J Mol Evol. 2024 Oct;92(5):593-604. doi: 10.1007/s00239-024-10199-4. Epub 2024 Sep 11. J Mol Evol. 2024. PMID: 39259330 Review.
-
Circular code motifs in the ribosome: a missing link in the evolution of translation?RNA. 2019 Dec;25(12):1714-1730. doi: 10.1261/rna.072074.119. Epub 2019 Sep 10. RNA. 2019. PMID: 31506380 Free PMC article.
References
-
- Bernier C, Petrov AS, Waterbury C, Jett J, Li F, Freil LE, Xiong B, Wang L, Le A, Milhouse BL, et al. 2014. Ribovision: visualization and analysis of ribosomes. Faraday Discuss. 169:195–207. - PubMed
-
- Bokov K, Steinberg SV.. 2009. A hierarchical model for evolution of 23S ribosomal RNA. Nature 457:977–980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

