Reduced T-Helper 17 Responses to Streptococcus pneumoniae in Infection-Prone Children Can Be Rescued by Addition of Innate Cytokines
- PMID: 28201637
- PMCID: PMC5441110
- DOI: 10.1093/infdis/jix090
Reduced T-Helper 17 Responses to Streptococcus pneumoniae in Infection-Prone Children Can Be Rescued by Addition of Innate Cytokines
Abstract
Background: T-helper (Th) 17 cells are important in the control of Streptococcus pneumoniae. We sought to understand the mechanism of failure of Th17 immunity resulting in S. pneumoniae infections in children <2 years old.
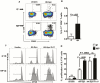
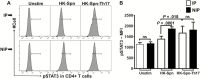
Methods: Peripheral blood mononuclear cells (PBMCs) from infection-prone (IP) and non-IP (NIP) children 9-18 months old were examined for their responses to heat-killed S. Pneumoniae, using flow cytometry, reverse-transcription polymerase chain reaction, and enzyme-linked immunoassay. We measured cytokine production, proliferation, and differentiation of Th17 cells and the expression of transcription factors in response to S. pneumoniae.
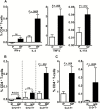
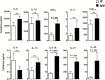
Results: PBMCs of IP children stimulated with heat-killed S. pneumoniae had significantly reduced percentages of CD4+ Th1 (interleukin2, tumor necrosis factor α) and Th17 (interleukin 17A) cells compared with NIP children. Addition of exogenous Th17-promoting cytokines (interleukin 6, 1β, and 23 and transforming growth factor β) restored CD4+ Th17 cell function in cells from IP children to levels measured in NIP children.
Conclusions: Reduced Th17 responses to S. pneumoniae in PBMCs of IP children can be rescued by addition of Th17-promoting cytokines.
Keywords: Pneumococcus; Th17; innate cytokines; young children..
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
-
- O’Brien KL, Wolfson LJ, Watt JP, et al. ; Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893–902. - PubMed
-
- Pichichero ME. Otitis media. Pediatr Clin North Am 2013; 60:391–407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

