Simultaneous processing and degradation of mitochondrial RNAs revealed by circularized RNA sequencing
- PMID: 28201688
- PMCID: PMC5435911
- DOI: 10.1093/nar/gkx104
Simultaneous processing and degradation of mitochondrial RNAs revealed by circularized RNA sequencing
Abstract
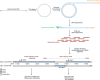
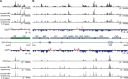
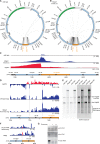
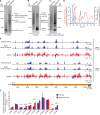
Mammalian mitochondrial RNAs are unique as they are derived from primary transcripts that encompass almost the entire mitochondrial genome. This necessitates extensive processing to release the individual mRNAs, rRNAs and tRNAs required for gene expression. Recent studies have revealed many of the proteins required for mitochondrial RNA processing, however the rapid turnover of precursor RNAs has made it impossible to analyze their composition and the hierarchy of processing. Here, we find that circularization of RNA prior to deep sequencing enables the discovery and characterization of unprocessed RNAs. Using this approach, we identify the most stable processing intermediates and the presence of intermediate processing products that are partially degraded and polyadenylated. Analysis of libraries constructed using RNA from mice lacking the nuclease subunit of the mitochondrial RNase P reveals the identities of stalled processing intermediates, their order of cleavage, and confirms the importance of RNase P in generating mature mitochondrial RNAs. Using RNA circularization prior to library preparation should provide a generally useful approach to studying RNA processing in many different biological systems.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Analysis of Mitochondrial RNA-Processing Defects in Patient-Derived Tissues by qRT-PCR and RNAseq.Methods Mol Biol. 2017;1567:379-390. doi: 10.1007/978-1-4939-6824-4_23. Methods Mol Biol. 2017. PMID: 28276031
-
RNA processing in human mitochondria.Cell Cycle. 2011 Sep 1;10(17):2904-16. doi: 10.4161/cc.10.17.17060. Epub 2011 Sep 1. Cell Cycle. 2011. PMID: 21857155
-
Investigating Mitochondrial Transcriptomes and RNA Processing Using Circular RNA Sequencing.Methods Mol Biol. 2021;2192:43-57. doi: 10.1007/978-1-0716-0834-0_4. Methods Mol Biol. 2021. PMID: 33230764
-
Maturation of 5' ends of plant mitochondrial RNAs.Physiol Plant. 2016 Jul;157(3):280-8. doi: 10.1111/ppl.12423. Epub 2016 Mar 23. Physiol Plant. 2016. PMID: 26833432 Review.
-
The post-transcriptional life of mammalian mitochondrial RNA.Biochem J. 2012 Jun 15;444(3):357-73. doi: 10.1042/BJ20112208. Biochem J. 2012. PMID: 22642575 Review.
Cited by
-
Full-length transcriptome profiling of Aphidius gifuensis mitochondrial genome with gene rearrangement and control region duplication.Heliyon. 2023 Jun 8;9(6):e17070. doi: 10.1016/j.heliyon.2023.e17070. eCollection 2023 Jun. Heliyon. 2023. PMID: 37484367 Free PMC article.
-
ANGEL2 phosphatase activity is required for non-canonical mitochondrial RNA processing.Nat Commun. 2022 Sep 30;13(1):5750. doi: 10.1038/s41467-022-33368-9. Nat Commun. 2022. PMID: 36180430 Free PMC article.
-
Defects of mitochondrial RNA turnover lead to the accumulation of double-stranded RNA in vivo.PLoS Genet. 2019 Jul 31;15(7):e1008240. doi: 10.1371/journal.pgen.1008240. eCollection 2019 Jul. PLoS Genet. 2019. PMID: 31365523 Free PMC article.
-
LRPPRC-mediated folding of the mitochondrial transcriptome.Nat Commun. 2017 Nov 16;8(1):1532. doi: 10.1038/s41467-017-01221-z. Nat Commun. 2017. PMID: 29146908 Free PMC article.
-
Manipulating and elucidating mitochondrial gene expression with engineered proteins.Philos Trans R Soc Lond B Biol Sci. 2020 Jan 20;375(1790):20190185. doi: 10.1098/rstb.2019.0185. Epub 2019 Dec 2. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31787043 Free PMC article. Review.
References
-
- Small I.D., Rackham O., Filipovska A.. Organelle transcriptomes: products of a deconstructed genome. Curr. Opin. Microbiol. 2013; 16:652–658. - PubMed
-
- Rackham O., Mercer T.R., Filipovska A.. The human mitochondrial transcriptome and the RNA-binding proteins that regulate its expression. Wiley Interdiscip. Rev. RNA. 2012; 3:675–695. - PubMed
-
- Hällberg B.M., Larsson N.-G.. Making proteins in the powerhouse. Cell Metab. 2014; 20:226–240. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

