Oxidative and nitrosative stress defences of Helicobacter and Campylobacter species that counteract mammalian immunity
- PMID: 28201757
- PMCID: PMC5091033
- DOI: 10.1093/femsre/fuw025
Oxidative and nitrosative stress defences of Helicobacter and Campylobacter species that counteract mammalian immunity
Abstract
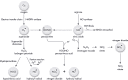
Helicobacter and Campylobacter species are Gram-negative microaerophilic host-associated heterotrophic bacteria that invade the digestive tract of humans and animals. Campylobacter jejuni is the major worldwide cause of foodborne gastroenteritis in humans, while Helicobacter pylori is ubiquitous in over half of the world's population causing gastric and duodenal ulcers. The colonisation of the gastrointestinal system by Helicobacter and Campylobacter relies on numerous cellular defences to sense the host environment and respond to adverse conditions, including those imposed by the host immunity. An important antimicrobial tool of the mammalian innate immune system is the generation of harmful oxidative and nitrosative stresses to which pathogens are exposed during phagocytosis. This review summarises the regulators, detoxifying enzymes and subversion mechanisms of Helicobacter and Campylobacter that ultimately promote the successful infection of humans.
Keywords: Helicobacter; Campylobacter; pathogen; innate immunity; bacterial defences.
Figures


References
-
- Alamuri P, Maier RJ. Methionine sulphoxide reductase is an important antioxidant enzyme in the gastric pathogen Helicobacter pylori. Mol Microbiol. 2004;53:1397–406. - PubMed
-
- Alfonso-Prieto M, Biarnes X, Vidossich P, et al. The molecular mechanism of the catalase reaction. J Am Chem Soc. 2009;131:11751–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

