Newborn Mice Lacking the Gene for Cyp1a1 Are More Susceptible to Oxygen-Mediated Lung Injury, and Are Rescued by Postnatal β-Naphthoflavone Administration: Implications for Bronchopulmonary Dysplasia in Premature Infants
- PMID: 28201809
- PMCID: PMC5837454
- DOI: 10.1093/toxsci/kfx036
Newborn Mice Lacking the Gene for Cyp1a1 Are More Susceptible to Oxygen-Mediated Lung Injury, and Are Rescued by Postnatal β-Naphthoflavone Administration: Implications for Bronchopulmonary Dysplasia in Premature Infants
Erratum in
-
Correction to: Newborn Mice Lacking the Gene for Cyp1a1 Are More Susceptible to Oxygen-Mediated Lung Injury, and Are Rescued by Postnatal β-Naphthoflavone Administration: Implications for Bronchopulmonary Dysplasia in Premature Infants.Toxicol Sci. 2022 Apr 26;187(1):187. doi: 10.1093/toxsci/kfac031. Toxicol Sci. 2022. PMID: 35348789 Free PMC article. No abstract available.
Abstract
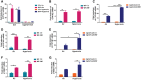
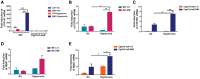
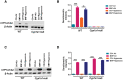
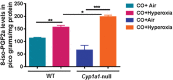
Prolonged hyperoxia contributes to bronchopulmonary dysplasia (BPD) in preterm infants. β-Naphthoflavone (BNF) is a potent inducer of cytochrome P450 (CYP)1A enzymes, which have been implicated in hyperoxic injuries in adult mice. In this investigation, we tested the hypothesis that newborn mice lacking the Cyp1a1 gene would be more susceptible to hyperoxic lung injury than wild-type (WT) mice and that postnatal BNF treatment would rescue this phenotype by mechanisms involving CYP1A and/or NAD(P)H quinone oxidoreductase (NQO1) enzymes. Newborn WT or Cyp1a1-null mice were treated with BNF (10 mg/kg) or the vehicle corn oil (CO) i.p., from postnatal day (PND) 2 to 14 once every other day, while being maintained in room air or hyperoxia (85% O2) for 14 days. Both genotypes showed lung injury, inflammation, and alveolar simplification in hyperoxia, with Cyp1a1-null mice displaying increased susceptibility compared to WT mice. BNF treatment resulted in significant attenuation of lung injury and inflammation, with improved alveolarization in both WT and Cyp1a1-null mice. BNF exposed normoxic or hyperoxic WT mice showed increased expression of hepatic CYP1A1/1A2, pulmonary CYP1A1, and NQO1 expression at both mRNA and protein levels, compared with vehicle controls. However, BNF caused greater induction of hepatic CYP1A2 and pulmonary NQO1 enzymes in the Cyp1a1-null mice, suggesting that BNF protects against hyperoxic lung injury in WT and Cyp1a1-null mice through the induction of CYP1A and NQO1 enzymes. Further studies on the protective role of flavonoids against hyperoxic lung injury in newborns could lead to novel strategies for the prevention and/or treatment of BPD.
Keywords: bronchopulmonary dysplasia.; cytochrome P4501A1; lung; newborn; oxidative injury; β-naphthoflavone.
© The Author 2017. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Anwar-Mohamed A., Abdelhamid G., Amara I. E., El-Kadi A. O. (2012). Differential modulation of aryl hydrocarbon receptor regulated enzymes by arsenite in the kidney, lung, and heart of C57BL/6 mice. Arch. Toxicol. 86, 897–910. - PubMed
-
- Brauze D., Zawierucha P., Kiwerska K., Bednarek K., Oleszak M., Rydzanicz M., Jarmuz-Szymczak M. (2017). Induction of expression of aryl hydrocarbon receptor-dependent genes in human HepaRG cell line modified by shRNA and treated with beta-naphthoflavone. Mol. Cell. Biochem. 425, 59–75. - PMC - PubMed
-
- Campbell S. J., Henderson C. J., Anthony D. C., Davidson D., Clark A. J., Wolf C. R. (2005). The murine Cyp1a1 gene is expressed in a restricted spatial and temporal pattern during embryonic development. J. Biol. Chem. 280, 5828–5835. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

