Targeted Contrast Agent Specific to an Oncoprotein in Tumor Microenvironment with the Potential for Detection and Risk Stratification of Prostate Cancer with MRI
- PMID: 28201871
- PMCID: PMC6075728
- DOI: 10.1021/acs.bioconjchem.6b00719
Targeted Contrast Agent Specific to an Oncoprotein in Tumor Microenvironment with the Potential for Detection and Risk Stratification of Prostate Cancer with MRI
Abstract
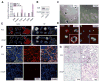
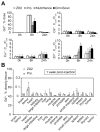
Accurate detection and risk stratification are paramount to the clinical management of prostate cancer. Current diagnostic methods, including prostate specific antigen (PSA) screening, are unable to differentiate high-risk tumors from low-risk tumors, resulting in overdiagnosis and overtreatment. A peptide targeted contrast agent, ZD2-Gd(HP-DO3A), specific to an oncoprotein in tumor microenvironment, EDB-FN, was synthesized for noninvasive detection and characterization of aggressive prostate cancer. EDB-FN, one of the subtypes of oncofetal fibronectin, is involved in tumor epithelial-to-mesenchymal transition (EMT), which is implicated in drug resistance and metastasis. The EDB-FN mRNA level in the metastatic PC3 cells was at least three times higher than that in non-metastatic LNCaP cells. In tumors, EDB-FN protein was highly expressed in PC3 tumor xenografts, but not in LNCaP tumors, as revealed by Western blot analysis. ZD2-Gd(HP-DO3A) produced over two times higher contrast-to-noise ratio in the PC3 tumors than in the LNCaP tumors in contrast-enhanced MRI during 30 min after injection. ZD2-Gd(HP-DO3A) possessed high chelate stability against transmetalation and minimal tissue accumulation. Our results demonstrate that molecular MRI of EDB-FN with ZD2-Gd(HP-DO3A) can potentially be used for noninvasive detection and risk stratification of human prostate cancer. Incorporation of this targeted contrast agent in the existing clinical contrast enhanced MRI procedures has the potential to improve diagnostic accuracy of prostate cancer.
Figures

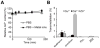



References
-
- Roobol M. Perspective: Enforce the clinical guidelines. Nature. 2015;528:S123. - PubMed
-
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P Clinical Guidelines Committee of the American College of P. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158:761–9. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Glass AS, Cary KC, Cooperberg MR. Risk-based prostate cancer screening: who and how? Curr Urol Rep. 2013;14:192–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

