High flavonoid accompanied with high starch accumulation triggered by nutrient starvation in bioenergy crop duckweed (Landoltia punctata)
- PMID: 28201992
- PMCID: PMC5310006
- DOI: 10.1186/s12864-017-3559-z
High flavonoid accompanied with high starch accumulation triggered by nutrient starvation in bioenergy crop duckweed (Landoltia punctata)
Abstract
Background: As the fastest growing plant, duckweed can thrive on anthropogenic wastewater. The purple-backed duckweed, Landoltia punctata, is rich in starch and flavonoids. However, the molecular biological basis of high flavonoid and low lignin content remains largely unknown, as does the best method to combine nutrients removed from sewage and the utilization value improvement of duckweed biomass.
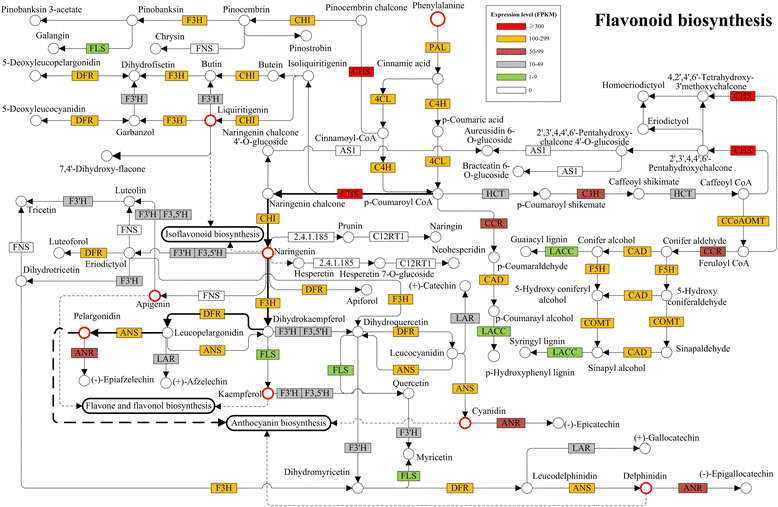
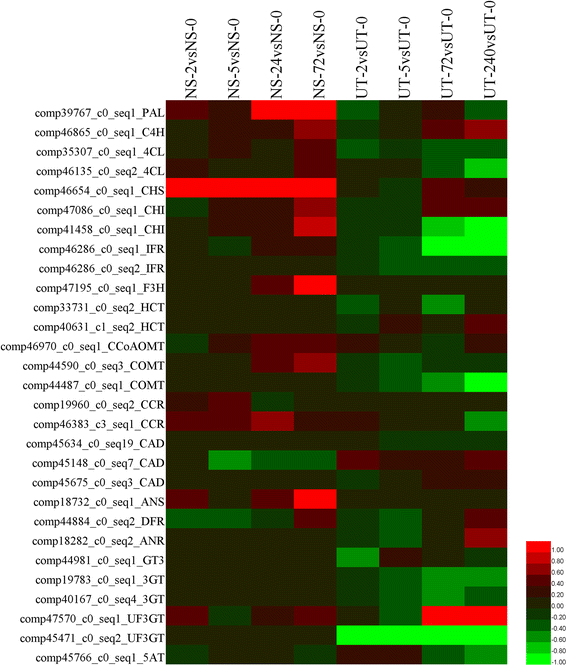
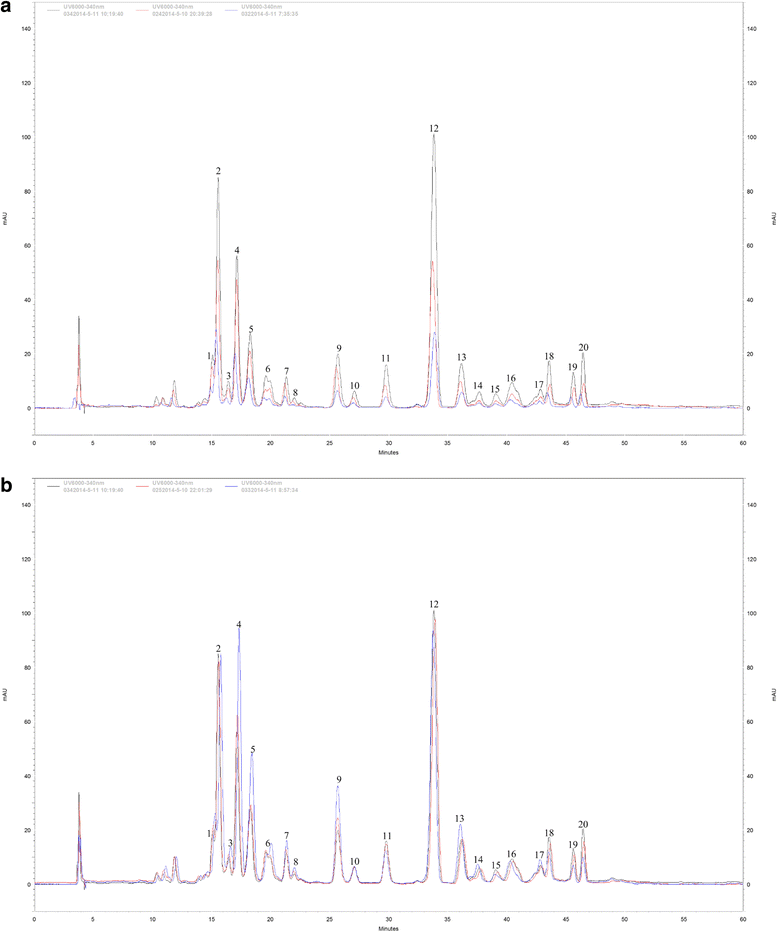
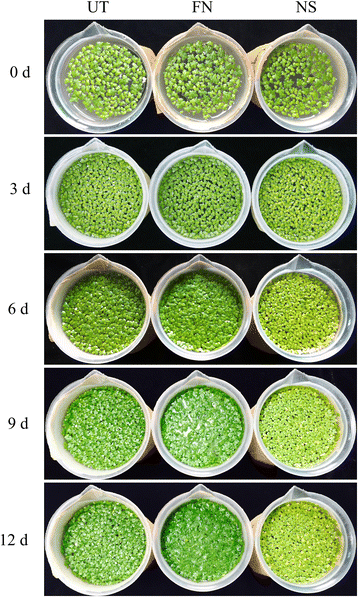
Results: A combined omics study was performed to investigate the biosynthesis of flavonoid and the metabolic flux changes in L. punctata grown in different culture medium. Phenylalanine metabolism related transcripts were identified and carefully analyzed. Expression quantification results showed that most of the flavonoid biosynthetic transcripts were relatively highly expressed, while most lignin-related transcripts were poorly expressed or failed to be detected by iTRAQ based proteomic analyses. This explains why duckweed has a much lower lignin percentage and higher flavonoid content than most other plants. Growing in distilled water, expression of most flavonoid-related transcripts were increased, while most were decreased in uniconazole treated L. punctata (1/6 × Hoagland + 800 mg•L-1 uniconazole). When L. punctata was cultivated in full nutrient medium (1/6 × Hoagland), more than half of these transcripts were increased, however others were suppressed. Metabolome results showed that a total of 20 flavonoid compounds were separated by HPLC in L. punctata grown in uniconazole and full nutrient medium. The quantities of all 20 compounds were decreased by uniconazole, while 11 were increased and 6 decreased when grown in full nutrient medium. Nutrient starvation resulted in an obvious purple accumulation on the underside of each frond.
Conclusions: The high flavonoid and low lignin content of L. punctata appears to be predominantly caused by the flavonoid-directed metabolic flux. Nutrient starvation is the best option to obtain high starch and flavonoid accumulation simultaneously in a short time for biofuels fermentation and natural products isolation.
Keywords: Combined omics; Duckweed; Flavonoids; Nutrient starvation; Starch; Uniconazole.
Figures


References
-
- Mazur WM, Duke JA, Wähälä K, Rasku S, Adlercreutz H. Isoflavonoids and lignans in legumes: nutritional and health aspects in humans. J Nutr Biochem. 1998;9(4):193–200. doi: 10.1016/S0955-2863(97)00184-8. - DOI
-
- Crowden R, Jarman S. 3-deoxyanthocyanins from the fern Blechnum procerum. Phytochemistry. 1974;13(9):1947–1948. doi: 10.1016/0031-9422(74)85122-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

