A novel Pfs38 protein complex on the surface of Plasmodium falciparum blood-stage merozoites
- PMID: 28202027
- PMCID: PMC5312596
- DOI: 10.1186/s12936-017-1716-0
A novel Pfs38 protein complex on the surface of Plasmodium falciparum blood-stage merozoites
Abstract
Background: The Plasmodium genome encodes for a number of 6-Cys proteins that contain a module of six cysteine residues forming three intramolecular disulphide bonds. These proteins have been well characterized at transmission as well as hepatic stages of the parasite life cycle. In the present study, a large complex of 6-Cys proteins: Pfs41, Pfs38 and Pfs12 and three other merozoite surface proteins: Glutamate-rich protein (GLURP), SERA5 and MSP-1 were identified on the Plasmodium falciparum merozoite surface.
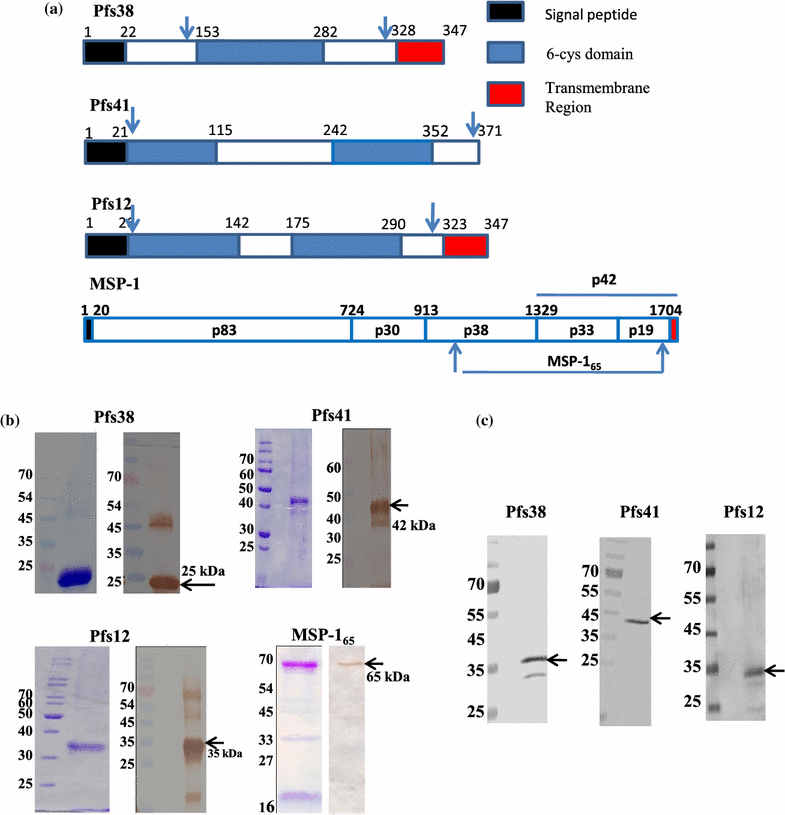
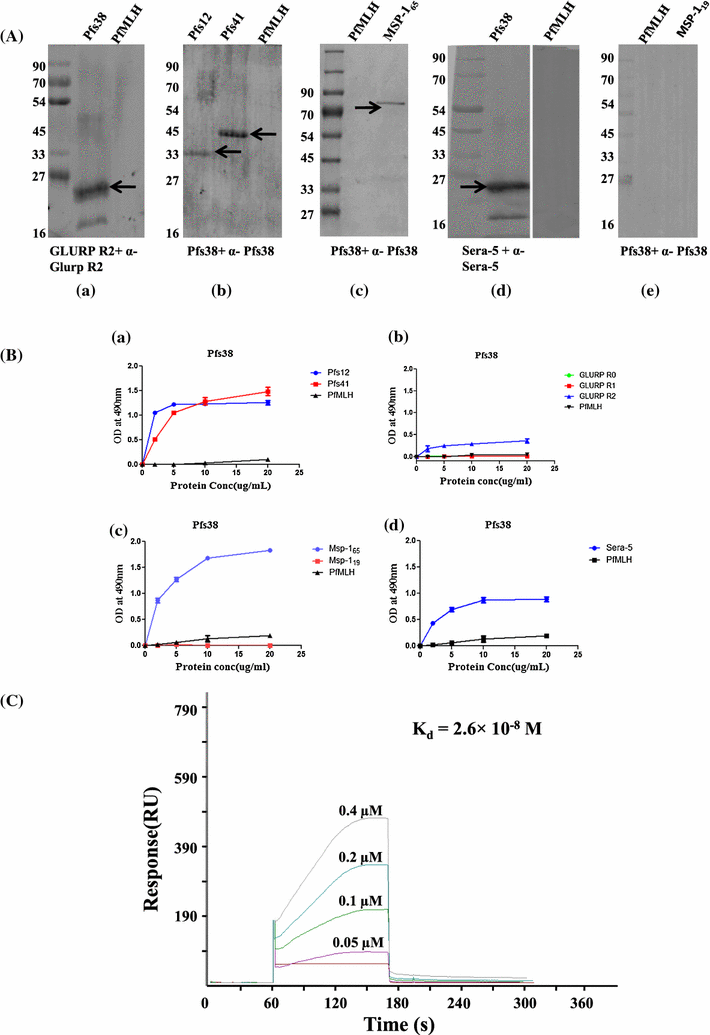
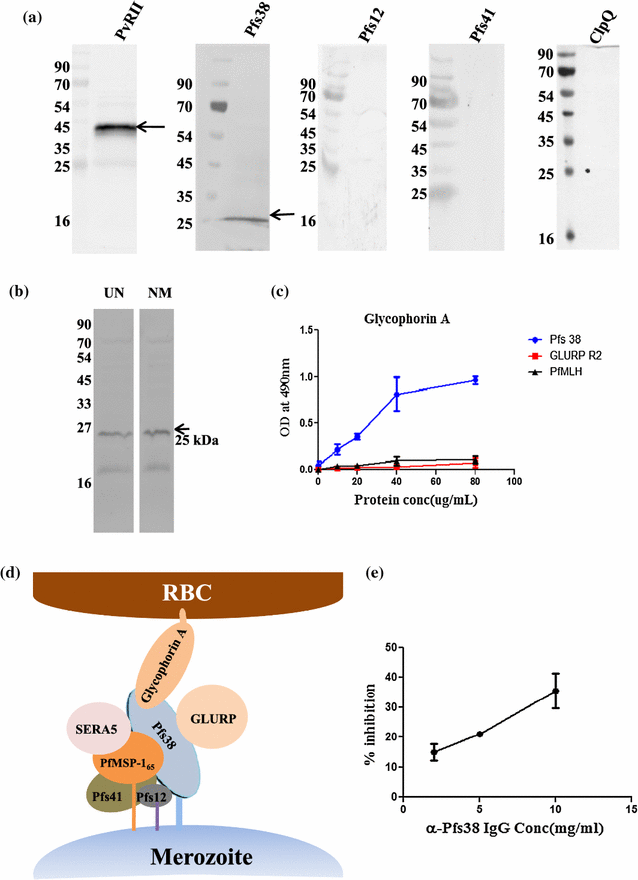
Methods: Recombinant 6-cys proteins i.e. Pfs38, Pfs12, Pfs41 as well as PfMSP-165 were expressed and purified using Escherichia coli expression system and antibodies were raised against each of these proteins. These antibodies were used to immunoprecipitate the native proteins and their associated partners from parasite lysate. ELISA, Far western, surface plasmon resonance and glycerol density gradient fractionation were carried out to confirm the respective interactions. Furthermore, erythrocyte binding assay with 6-cys proteins were undertaken to find out their possible role in host-parasite infection and seropositivity was assessed using Indian and Liberian sera.
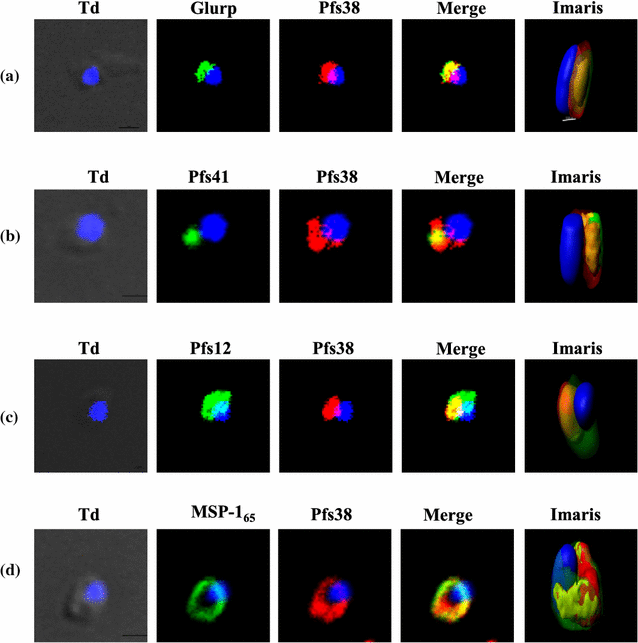
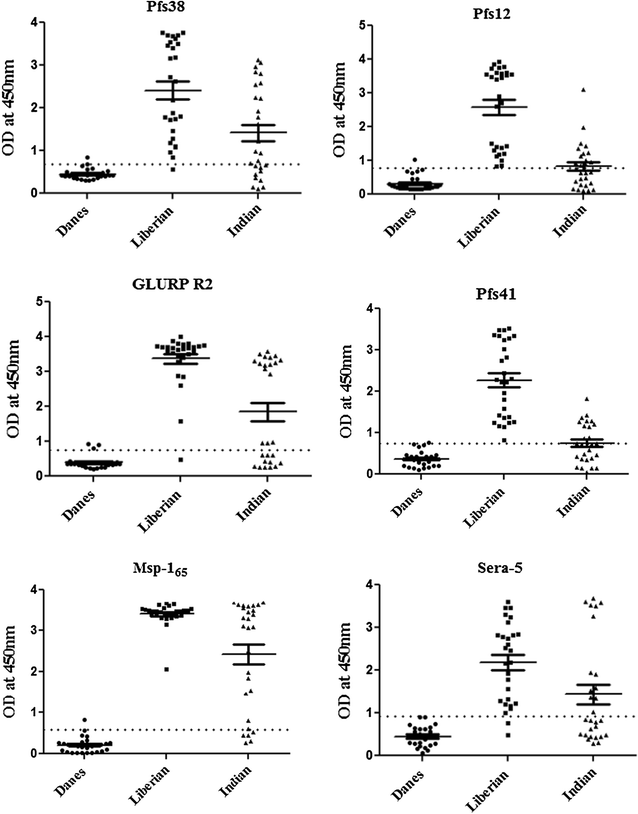
Results: Immunoprecipitation of parasite-derived polypeptides, followed by LC-MS/MS analysis, identified a large Pfs38 complex comprising of 6-cys proteins: Pfs41, Pfs38, Pfs12 and other merozoite surface proteins: GLURP, SERA5 and MSP-1. The existence of such a complex was further corroborated by several protein-protein interaction tools, co-localization and co-sedimentation analysis. Pfs38 protein of Pfs38 complex binds to host red blood cells (RBCs) directly via glycophorin A as a receptor. Seroprevalence analysis showed that of the six antigens, prevalence varied from 40 to 99%, being generally highest for MSP-165 and GLURP proteins.
Conclusions: Together the data show the presence of a large Pfs38 protein-associated complex on the parasite surface which is involved in RBC binding. These results highlight the complex molecular interactions among the P. falciparum merozoite surface proteins and advocate the development of a multi-sub-unit malaria vaccine based on some of these protein complexes on merozoite surface.
Keywords: 6-cys proteins; Glycophorin A; MSP-165; Plasmodium falciparum.
Figures
Similar articles
-
Plasmodium falciparum MSP3 Exists in a Complex on the Merozoite Surface and Generates Antibody Response during Natural Infection.Infect Immun. 2018 Jul 23;86(8):e00067-18. doi: 10.1128/IAI.00067-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29760216 Free PMC article.
-
Proteome analysis reveals a large merozoite surface protein-1 associated complex on the Plasmodium falciparum merozoite surface.J Proteome Res. 2011 Feb 4;10(2):680-91. doi: 10.1021/pr100875y. Epub 2010 Dec 22. J Proteome Res. 2011. PMID: 21175202
-
A library of functional recombinant cell-surface and secreted P. falciparum merozoite proteins.Mol Cell Proteomics. 2013 Dec;12(12):3976-86. doi: 10.1074/mcp.O113.028357. Epub 2013 Sep 16. Mol Cell Proteomics. 2013. PMID: 24043421 Free PMC article.
-
Antigenic Variation in Plasmodium falciparum.Results Probl Cell Differ. 2015;57:47-90. doi: 10.1007/978-3-319-20819-0_3. Results Probl Cell Differ. 2015. PMID: 26537377 Review.
-
Plasmodium vivax: a glimpse into the unique and shared biology of the merozoite.Ann Trop Med Parasitol. 1995 Apr;89(2):113-20. doi: 10.1080/00034983.1995.11812941. Ann Trop Med Parasitol. 1995. PMID: 7605120 Review.
Cited by
-
Proteome-wide comparison of tertiary protein structures reveals molecular mimicry in Plasmodium-human interactions.Front Parasitol. 2023 Jun 15;2:1162697. doi: 10.3389/fpara.2023.1162697. eCollection 2023. Front Parasitol. 2023. PMID: 39816809 Free PMC article.
-
Size and sequence polymorphisms in the glutamate-rich protein gene of the human malaria parasite Plasmodium falciparum in Thailand.Parasit Vectors. 2018 Jan 22;11(1):49. doi: 10.1186/s13071-018-2630-1. Parasit Vectors. 2018. PMID: 29357909 Free PMC article.
-
Plasmodium falciparum MSP3 Exists in a Complex on the Merozoite Surface and Generates Antibody Response during Natural Infection.Infect Immun. 2018 Jul 23;86(8):e00067-18. doi: 10.1128/IAI.00067-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29760216 Free PMC article.
-
Plasmodium falciparum Clag9-Associated PfRhopH Complex Is Involved in Merozoite Binding to Human Erythrocytes.Infect Immun. 2020 Jan 22;88(2):e00504-19. doi: 10.1128/IAI.00504-19. Print 2020 Jan 22. Infect Immun. 2020. PMID: 31712270 Free PMC article.
-
An essential vesicular-trafficking phospholipase mediates neutral lipid synthesis and contributes to hemozoin formation in Plasmodium falciparum.BMC Biol. 2021 Aug 11;19(1):159. doi: 10.1186/s12915-021-01042-z. BMC Biol. 2021. PMID: 34380472 Free PMC article.
References
-
- WHO. World malaria report. Geneva: World Health Organization; 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report....
-
- Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–827. doi: 10.1086/339342. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

