In vitro generation of Sertoli-like and haploid spermatid-like cells from human umbilical cord perivascular cells
- PMID: 28202061
- PMCID: PMC5312448
- DOI: 10.1186/s13287-017-0491-8
In vitro generation of Sertoli-like and haploid spermatid-like cells from human umbilical cord perivascular cells
Abstract
Background: First trimester (FTM) and term human umbilical cord-derived perivascular cells (HUCPVCs), which are rich sources of mesenchymal stem cells (MSCs), can give rise to Sertoli cell (SC)-like as well as haploid germ cell (GC)-like cells in vitro using culture conditions that recapitulate the testicular niche. Gamete-like cells have been produced ex vivo using pluripotent stem cells as well as MSCs. However, the production of functional gametes from human stem cells has yet to be achieved.
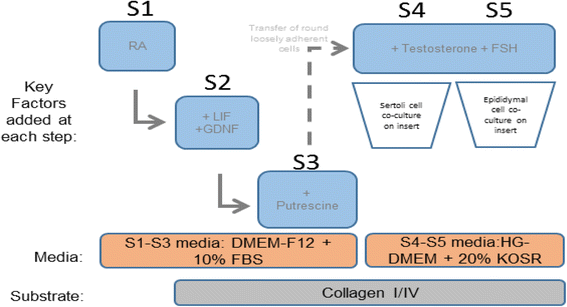
Methods: Three independent lines of FTM and term HUCPVCs were cultured using a novel 5-week step-wise in vitro differentiation protocol recapitulating key physiological signals involved in testicular development. SC- and GC-associated phenotypical properties were assessed by real-time polymerase chain reaction (RT-PCR), quantitative PCR immunocytochemistry, flow cytometry, and fluorescence in-situ hybridization (FISH). Functional spermatogonial stem cell-like properties were assessed using a xenotranplantation assay.
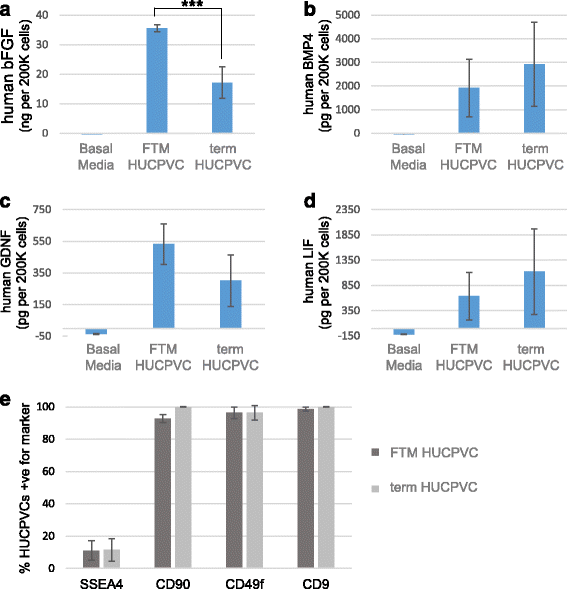
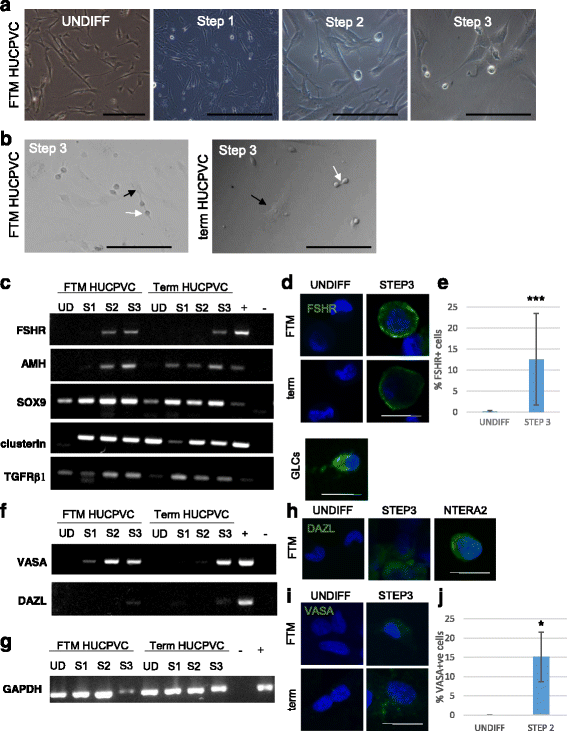
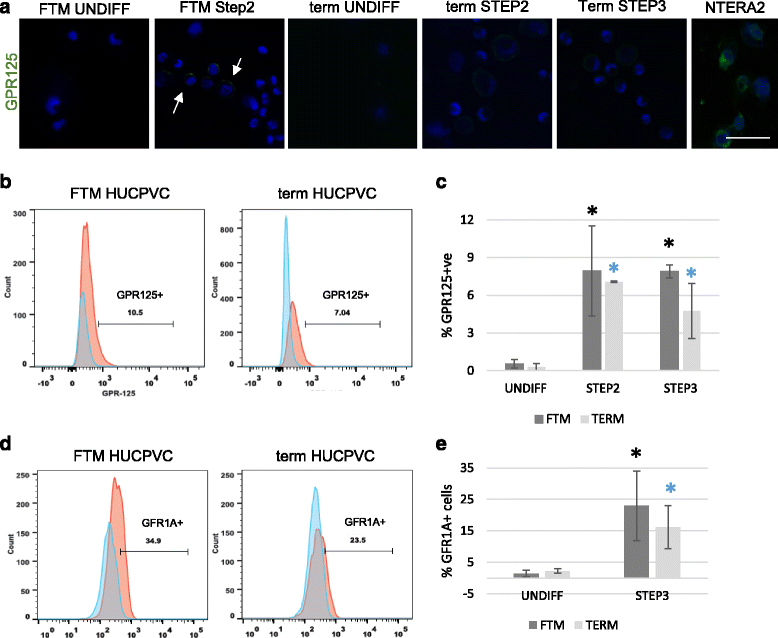
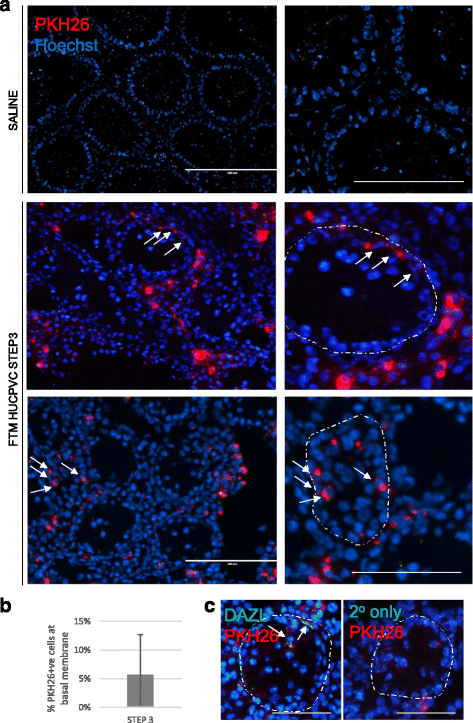
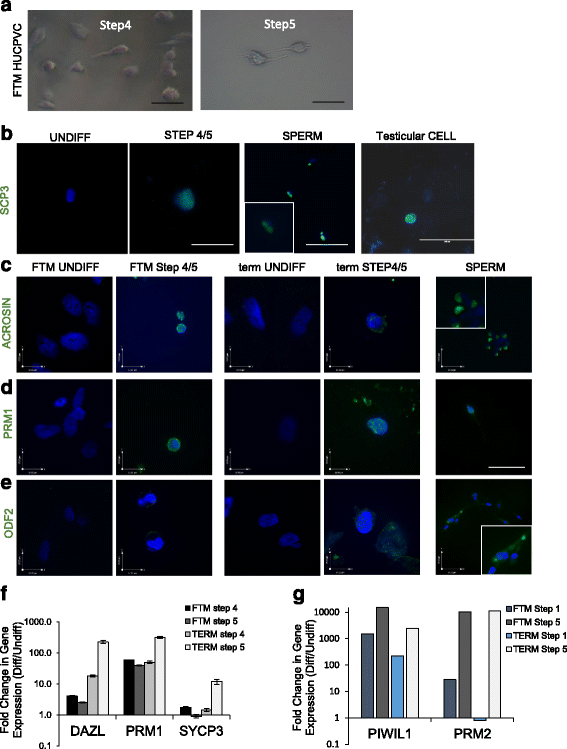
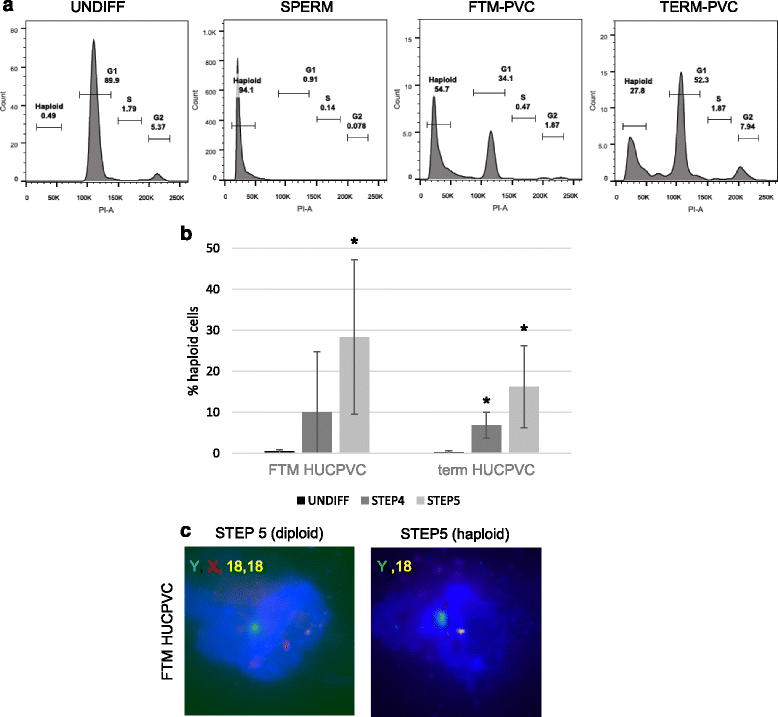
Results: Within 3 weeks of differentiation, two morphologically distinct cell types emerged including large adherent cells and semi-attached round cells. Both early GC-associated markers (VASA, DAZL, GPR125, GFR1α) and SC-associated markers (FSHR, SOX9, AMH) were upregulated, and 5.7 ± 1.2% of these cells engrafted near the inner basal membrane in a xenograft assay. After 5 weeks in culture, 10-30% of the cells were haploid, had adopted a spermatid-like morphology, and expressed PRM1, Acrosin, and ODF2. Undifferentiated HUCPVCs secreted key factors known to regulate spermatogenesis (LIF, GDNF, BMP4, bFGF) and 10-20% of HUCPVCs co-expressed SSEA4, CD9, CD90, and CD49f. We hypothesize that the paracrine properties and cellular heterogeneity of HUCPVCs may explain their dual capacity to differentiate to both SC- and GC-like cells.
Conclusions: HUCPVCs recapitulate elements of the testicular niche including their ability to differentiate into cells with Sertoli-like and haploid spermatid-like properties in vitro. Our study supports the importance of generating a niche-like environment under ex vivo conditions aiming at creating mature GC, and highlights the plasticity of HUCPVCs. This could have future applications for the treatment of some cases of male infertility.
Keywords: Ex-vivo spermatogenesis; Germ cell; Human mesenchymal stem cell; Human umbilical cord; Niche; Paracrine; Perivascular cell; Sertoli cell; Spermatogonial stem cell; Xenograft.
Figures
Similar articles
-
Human umbilical perivascular cells: a novel source of MSCs to support testicular niche regeneration.Reproduction. 2016 Oct 25:REP-16-0220. doi: 10.1530/REP-16-0220. Online ahead of print. Reproduction. 2016. PMID: 27780883
-
Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue.Mol Hum Reprod. 2017 Nov 1;23(11):738-754. doi: 10.1093/molehr/gax054. Mol Hum Reprod. 2017. PMID: 29040674
-
Dynamics, ultrastructure and gene expression of human in vitro organized testis cells from testicular sperm extraction biopsies.Mol Hum Reprod. 2018 Mar 1;24(3):123-134. doi: 10.1093/molehr/gax070. Mol Hum Reprod. 2018. PMID: 29304256
-
Potential use of stem cells for fertility preservation.Andrology. 2020 Jul;8(4):862-878. doi: 10.1111/andr.12713. Epub 2019 Oct 29. Andrology. 2020. PMID: 31560823 Review.
-
Mechanisms of hormonal regulation of sertoli cell development and proliferation: a key process for spermatogenesis.Curr Mol Pharmacol. 2014;7(2):96-108. doi: 10.2174/1874467208666150126155032. Curr Mol Pharmacol. 2014. PMID: 25620228 Review.
Cited by
-
Spermatogonial stem cell transplantation and male infertility: Current status and future directions.Arab J Urol. 2017 Dec 27;16(1):171-180. doi: 10.1016/j.aju.2017.11.015. eCollection 2018 Mar. Arab J Urol. 2017. PMID: 29713548 Free PMC article. Review.
-
Spermatogenesis Recovery Potentials after Transplantation of Adipose Tissue-Derived Mesenchymal Stem Cells Cultured with Growth Factors in Experimental Azoospermic Mouse Models.Cell J. 2020 Jan;21(4):401-409. doi: 10.22074/cellj.2020.6055. Epub 2019 Jul 29. Cell J. 2020. PMID: 31376321 Free PMC article.
-
Male germline stem cells in non-human primates.Primate Biol. 2017 Sep 22;4(2):173-184. doi: 10.5194/pb-4-173-2017. eCollection 2017. Primate Biol. 2017. PMID: 32110705 Free PMC article. Review.
-
In vivo and in vitro sperm production: an overview of the challenges and advances in male fertility restoration.Clin Exp Reprod Med. 2024 Sep;51(3):171-180. doi: 10.5653/cerm.2023.06569. Epub 2024 Mar 25. Clin Exp Reprod Med. 2024. PMID: 38525520 Free PMC article.
-
Multipotent fetal stem cells in reproductive biology research.Stem Cell Res Ther. 2023 Jun 7;14(1):157. doi: 10.1186/s13287-023-03379-4. Stem Cell Res Ther. 2023. PMID: 37287077 Free PMC article. Review.
References
-
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–26. doi: 10.1016/j.stem.2012.07.017. - DOI - PMC - PubMed
-
- Chuang CY, Lin KI, Hsiao M, Stone L, Chen HF, Huang YH, Lin SP, Ho HN, Kuo HC. Meiotic competent human germ cell-like cells derived from human embryonic stem cells induced by BMP4/WNT3A signaling and OCT4/EpCAM (epithelial cell adhesion molecule) selection. J Biol Chem. 2012;287(18):14389–401. doi: 10.1074/jbc.M111.338434. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

