The DNA damage response promotes polyomavirus JC infection by nucleus to cytoplasm NF- kappaB activation
- PMID: 28202068
- PMCID: PMC5312431
- DOI: 10.1186/s12985-017-0707-7
The DNA damage response promotes polyomavirus JC infection by nucleus to cytoplasm NF- kappaB activation
Abstract
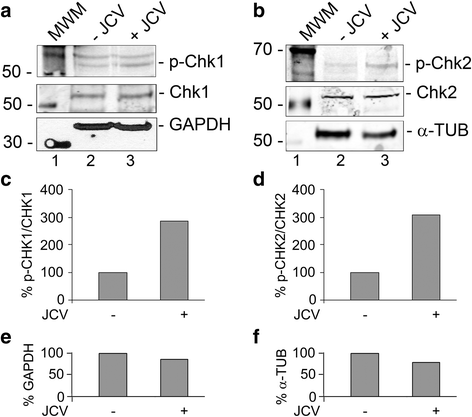
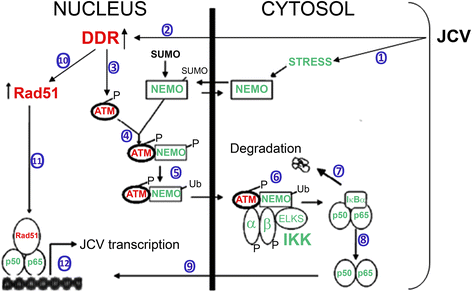
Background: Infection of glial cells by human neurotropic polyomavirus JC (JCV), the causative agent of the CNS demyelinating disease progressive multifocal leukoencephalopathy (PML), rapidly inflicts damage to cellular DNA. This activates DNA damage response (DDR) signaling including induction of expression of DNA repair factor Rad51. We previously reported that Rad51 co-operates with the transcription factor NF-κB p65 to activate JCV early transcription. Thus Rad51 induction by JCV infection may provide positive feedback for viral activation early in JCV infection. DDR is also known to stimulate NF-κB activity, a phenomenon known as nucleus to cytoplasm or "inside-out" NF-κB signaling, which is initiated by Ataxia telangiectasia mutated (ATM) protein, a serine/threonine kinase recruited and activated by DNA double-strand breaks. Downstream of ATM, there occurs a series of post-translational modifications of NF-κB essential modulator (NEMO), the γ regulatory subunit of inhibitor of NF-κB (IκB) kinase (IKK), resulting in NF-κB activation.
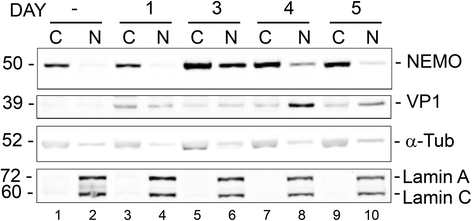
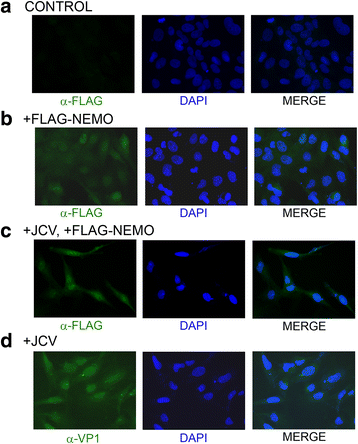
Methods: We analyzed the effects of downstream pathways in the DDR by phosphospecific Western blots and analysis of the subcellular distribution of NEMO by cell fractionation and immunocytochemistry. The role of DDR in JCV infection was analyzed using a small molecule inhibitor of ATM (KU-55933). NEMO sumoylation was investigated by Western and association of ATM and NEMO by immunoprecipitation/Western blots.
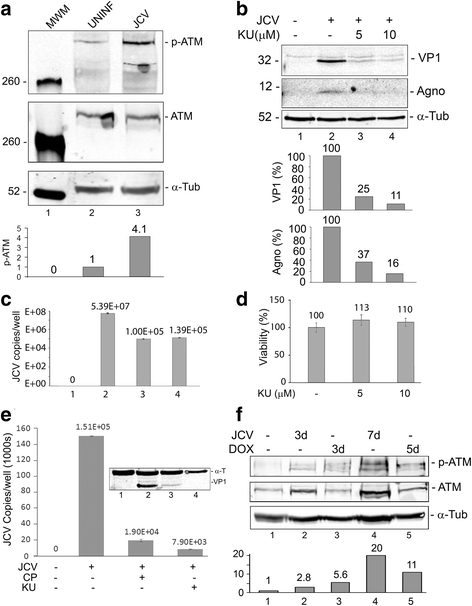
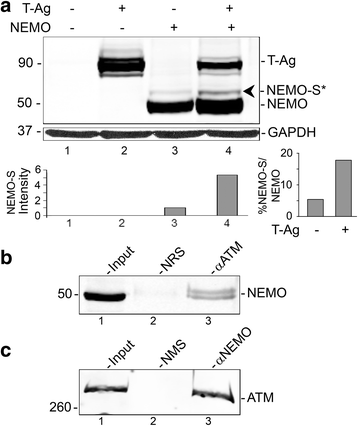
Results: We show that JCV infection caused phosphorylation and activation of ATM while KU-55933 inhibited JCV replication. JCV infection caused a redistribution of NEMO from cytoplasm to nucleus. Co-expression of JCV large T-antigen and FLAG-tagged NEMO showed the occurrence of sumoylation of NEMO, while co-expression of ATM and FLAG-NEMO demonstrated physical association between ATM and NEMO.
Conclusions: We propose a model where JCV infection induces both overexpression of Rad51 protein and activation of the nucleus to cytoplasm NF-κB signaling pathway, which then act together to enhance JCV gene expression.
Keywords: DNA damage response; Nuclear factor kappa-B; Polyomavirus JC; Progressive multifocal leukoencephalopathy.
Figures
Similar articles
-
Rad51 activates polyomavirus JC early transcription.PLoS One. 2014 Oct 13;9(10):e110122. doi: 10.1371/journal.pone.0110122. eCollection 2014. PLoS One. 2014. PMID: 25310191 Free PMC article.
-
HIV-1 Vpr-induced DNA damage activates NF-κB through ATM-NEMO independent of cell cycle arrest.mBio. 2024 Oct 16;15(10):e0024024. doi: 10.1128/mbio.00240-24. Epub 2024 Sep 13. mBio. 2024. PMID: 39269169 Free PMC article.
-
The Brd4 acetyllysine-binding protein is involved in activation of polyomavirus JC.J Neurovirol. 2016 Oct;22(5):615-625. doi: 10.1007/s13365-016-0435-6. Epub 2016 Mar 23. J Neurovirol. 2016. PMID: 27007123 Free PMC article.
-
DNA damage-dependent NF-κB activation: NEMO turns nuclear signaling inside out.Immunol Rev. 2012 Mar;246(1):311-26. doi: 10.1111/j.1600-065X.2012.01101.x. Immunol Rev. 2012. PMID: 22435563 Free PMC article. Review.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
Human polyomavirus modulation of the host DNA damage response.Virus Genes. 2020 Apr;56(2):128-135. doi: 10.1007/s11262-020-01736-6. Epub 2020 Jan 29. Virus Genes. 2020. PMID: 31997082 Review.
-
Brincidofovir inhibits polyomavirus infection in vivo.mBio. 2024 Aug 14;15(8):e0104924. doi: 10.1128/mbio.01049-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953354 Free PMC article.
-
Human Polyomaviruses: The Battle of Large and Small Tumor Antigens.Virology (Auckl). 2017 Dec 5;8:1178122X17744785. doi: 10.1177/1178122X17744785. eCollection 2017. Virology (Auckl). 2017. PMID: 29238174 Free PMC article. Review.
-
JCPyV-Induced MAPK Signaling Activates Transcription Factors during Infection.Int J Mol Sci. 2019 Sep 26;20(19):4779. doi: 10.3390/ijms20194779. Int J Mol Sci. 2019. PMID: 31561471 Free PMC article.
-
A multi-omic investigation of male lower urinary tract symptoms: Potential role for JC virus.PLoS One. 2021 Feb 25;16(2):e0246266. doi: 10.1371/journal.pone.0246266. eCollection 2021. PLoS One. 2021. PMID: 33630889 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

