Use of ethanol extracts of Terminalia chebula to prevent periodontal disease induced by dental plaque bacteria
- PMID: 28202081
- PMCID: PMC5312597
- DOI: 10.1186/s12906-017-1619-1
Use of ethanol extracts of Terminalia chebula to prevent periodontal disease induced by dental plaque bacteria
Abstract
Background: The fruit of the Terminalia chebula tree has been widely used for the treatment of various disorders. Its anti-diabetic, anti-mutagenic, anti-oxidant, anti-bacterial, anti-fungal, and anti-viral effects have been studied. Dental plaque bacteria (DPB) are intimately associated with gingivitis and periodontitis. In the quest for materials that will prove useful in the treatment and prevention of periodontal disease, we investigated the preventive effects of an ethanol extract of Terminalia chebula (EETC) on DPB-induced inflammation and bone resorption.
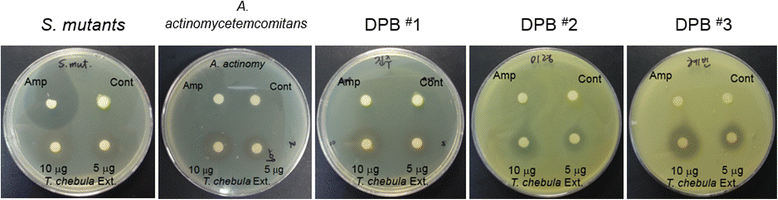
Methods: The anti-bacterial effect of EETC was analyzed using the disc diffusion method. The anti-inflammatory effect of EETC was determined by molecular biological analysis of the DPB-mediated culture cells. Prevention of osteoclastic bone resorption by EETC was explored using osteoclast formation and pit formation assays.
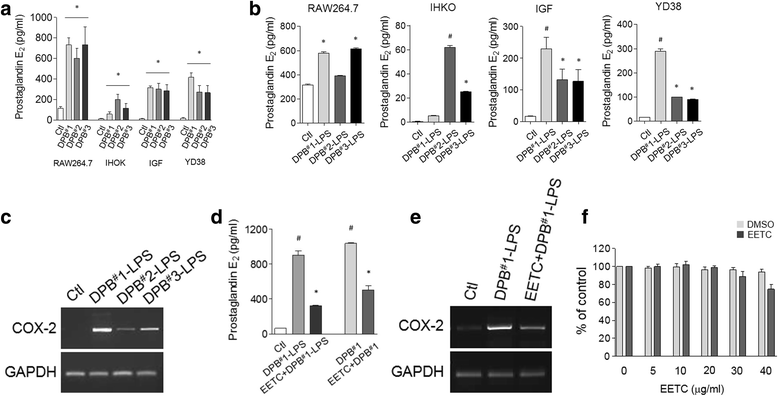
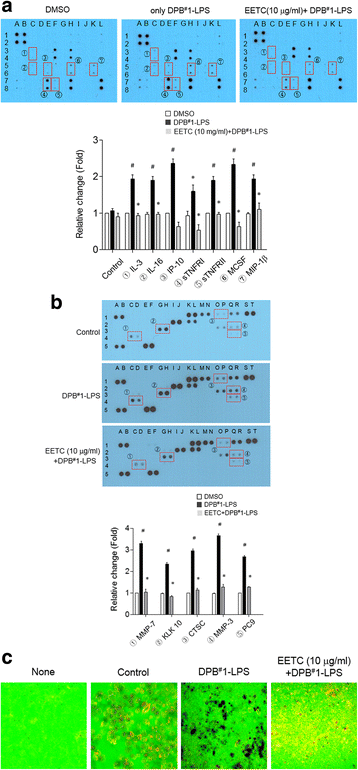
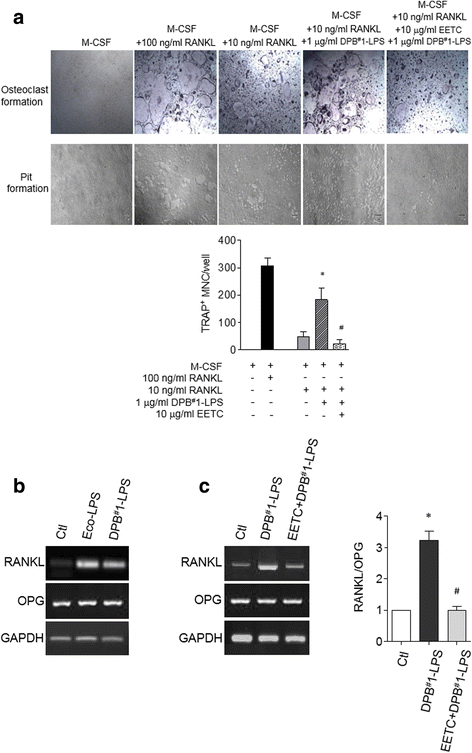
Results: EETC suppressed the growth of oral bacteria and reduced the induction of inflammatory cytokines and proteases, abolishing the expression of PGE2 and COX-2 and inhibiting matrix damage. By stimulating the DPB-derived lipopolysaccharides, EETC inhibited both osteoclast formation in osteoclast precursors and RANKL expression in osteoblasts, thereby contributing to the prevention of bone resorption.
Conclusions: EETC may be a beneficial supplement to help prevent DPB-mediated periodontal disease.
Keywords: Dental plaque bacteria (DPB); Ethanol extract of Terminalia chebula (EETC); Gingivitis; Inflammation; Lipopolysaccharide (LPS); Osteoclast; Periodontitis.
Figures
Similar articles
-
Anti-Periodontitis Effect of Ethanol Extracts of Alpinia Katsumadai Seeds.Nutrients. 2021 Dec 28;14(1):136. doi: 10.3390/nu14010136. Nutrients. 2021. PMID: 35011011 Free PMC article.
-
Antibacterial and antioxidant effect of ethanol extracts of Terminalia chebula on Streptococcus mutans.Clin Exp Dent Res. 2021 Dec;7(6):987-994. doi: 10.1002/cre2.467. Epub 2021 Jun 28. Clin Exp Dent Res. 2021. PMID: 34184430 Free PMC article.
-
Aminothiazoles inhibit osteoclastogenesis and PGE2 production in LPS-stimulated co-cultures of periodontal ligament and RAW 264.7 cells, and RANKL-mediated osteoclastogenesis and bone resorption in PBMCs.J Cell Mol Med. 2019 Feb;23(2):1152-1163. doi: 10.1111/jcmm.14015. Epub 2018 Dec 1. J Cell Mol Med. 2019. PMID: 30506812 Free PMC article.
-
Microbiology of periodontal disease -- present status and future considerations.J Periodontol. 1977 Sep;48(9):497-504. doi: 10.1902/jop.1977.48.9.497. J Periodontol. 1977. PMID: 333085 Review. No abstract available.
-
Pharmacologic management of periodontal diseases using systemically administered agents.Dent Clin North Am. 1998 Apr;42(2):245-62. Dent Clin North Am. 1998. PMID: 9597336 Review.
Cited by
-
Bibliometric Study of Adaptogens in Dermatology: Pharmacophylogeny, Phytochemistry, and Pharmacological Mechanisms.Drug Des Devel Ther. 2023 Feb 6;17:341-361. doi: 10.2147/DDDT.S395256. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 36776447 Free PMC article. Review.
-
Green Alternatives as Antimicrobial Agents in Mitigating Periodontal Diseases: A Narrative Review.Microorganisms. 2023 May 11;11(5):1269. doi: 10.3390/microorganisms11051269. Microorganisms. 2023. PMID: 37317243 Free PMC article. Review.
-
Autoclavable Albumin-Based Cryogels with Uncompromising Properties.Gels. 2023 Sep 1;9(9):712. doi: 10.3390/gels9090712. Gels. 2023. PMID: 37754393 Free PMC article.
-
Evaluation of In Vitro Antiprotease Activity of Selected Traditional Medicinal Herbs in Dentistry and Its In Silico PASS Prediction.Biomed Res Int. 2022 Jun 6;2022:5870443. doi: 10.1155/2022/5870443. eCollection 2022. Biomed Res Int. 2022. PMID: 35707383 Free PMC article.
-
Plants of the Genus Terminalia: An Insight on Its Biological Potentials, Pre-Clinical and Clinical Studies.Front Pharmacol. 2020 Oct 8;11:561248. doi: 10.3389/fphar.2020.561248. eCollection 2020. Front Pharmacol. 2020. PMID: 33132909 Free PMC article. Review.
References
-
- Sanz M, van Winkelhoff AJ, Herrera D, Dellemijn-Kippuw N, Simón R, Winkel E. Differences in the composition of the subgingival microbiota of two periodontitis populations of different geographical origin. A comparison between Spain and the Netherlands. Eur J Oral Sci. 2000;108(5):383–92. doi: 10.1034/j.1600-0722.2000.108005383.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

