Aprepitant limits in vivo neuroinflammatory responses in a rhesus model of Lyme neuroborreliosis
- PMID: 28202084
- PMCID: PMC5312540
- DOI: 10.1186/s12974-017-0813-x
Aprepitant limits in vivo neuroinflammatory responses in a rhesus model of Lyme neuroborreliosis
Abstract
Background: Substance P (SP) is produced at high levels in the central nervous system (CNS), and its target receptor, neurokinin 1 receptor (NK-1R), is expressed by glia and leukocytes. This tachykinin functions to exacerbate inflammatory responses at peripheral sites. Moreover, SP/NK-1R interactions have recently been associated with severe neuroinflammation and neuronal damage. We have previously demonstrated that NK-1R antagonists can limit neuroinflammatory damage in a mouse model of bacterial meningitis. Furthermore, we have since shown that these agents can attenuate Borrelia burgdorferi-induced neuronal and glial inflammatory mediator production in non-human primate brain explants and isolated neuronal cells.
Methods: In the present study, we have assessed the role played by endogenous SP/NK-1R interactions in damaging CNS inflammation in an established rhesus macaque model that faithfully reproduces the key clinical features of Lyme neuroborreliosis, using the specific NK-1R antagonist, aprepitant. We have utilized multiplex ELISA to quantify immune mediator levels in cerebrospinal fluid, and RT-PCR and immunoblot analyses to quantify cytokine and NK-1R expression, respectively, in brain cortex, dorsal root ganglia, and spinal cord tissues. In addition, we have assessed astrocyte number/activation status in brain cortical tissue by immunofluorescence staining and confocal microscopy.
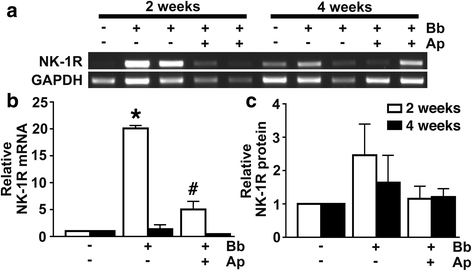
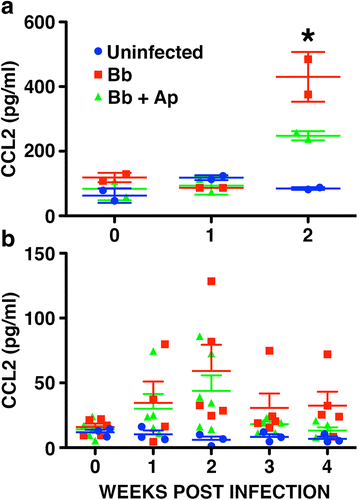
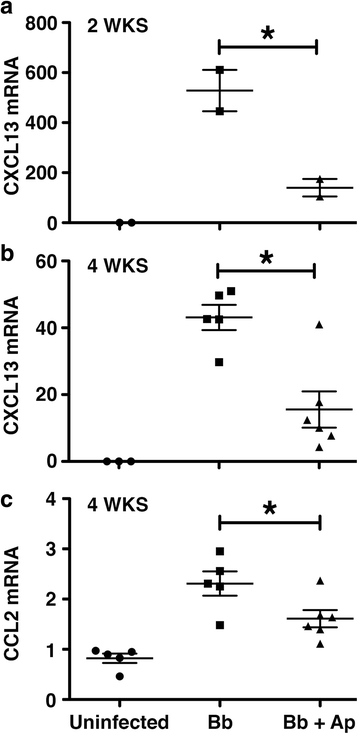
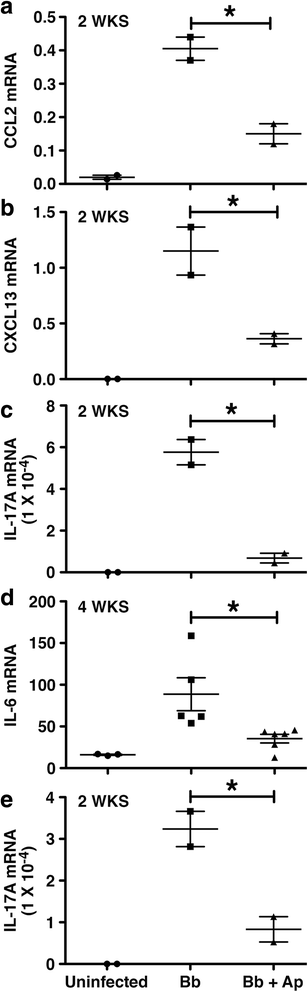
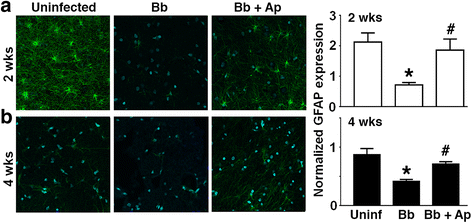
Results: We demonstrate that aprepitant treatment attenuates B. burgdorferi-induced elevations in CCL2, CXCL13, IL-17A, and IL-6 gene expression in dorsal root ganglia, spinal cord, and/or cerebrospinal fluid of rhesus macaques at 2 to 4 weeks following intrathecal infection. In addition, we demonstrate that this selective NK-1R antagonist also prevents increases in total cortical brain NK-1R expression and decreases in the expression of the astrocyte marker, glial fibrillary acidic protein, associated with B. burgdorferi infection.
Conclusions: The ability of a centrally acting NK-1R inhibitor to attenuate B. burgdorferi-associated neuroinflammatory responses and sequelae raises the intriguing possibility that such FDA-approved agents could be repurposed for use as an adjunctive therapy for the treatment of bacterial CNS infections.
Keywords: Borrelia burgdorferi; Lyme neuroborreliosis; NK-1R antagonist; Neuroinflammation; Substance P.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

