Deficiency of the Angiotensinase Aminopeptidase A Increases Susceptibility to Glomerular Injury
- PMID: 28202497
- PMCID: PMC5491295
- DOI: 10.1681/ASN.2016111166
Deficiency of the Angiotensinase Aminopeptidase A Increases Susceptibility to Glomerular Injury
Abstract
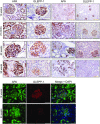
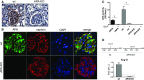
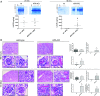
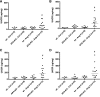
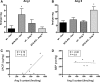
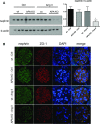
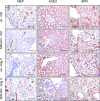
Aminopeptidase A (APA) is expressed in glomerular podocytes and tubular epithelia and metabolizes angiotensin II (AngII), a peptide known to promote glomerulosclerosis. In this study, we tested whether APA expression changes in response to progressive nephron loss or whether APA exerts a protective role against glomerular damage and during AngII-mediated hypertensive kidney injury. At advanced stages of FSGS, fawn-hooded hypertensive rat kidneys exhibited distinctly increased APA staining in areas of intact glomerular capillary loops. Moreover, BALB/c APA-knockout (KO) mice injected with a nephrotoxic serum showed persistent glomerular hyalinosis and albuminuria 96 hours after injection, whereas wild-type controls achieved virtually full recovery. We then tested the effect of 4-week infusion of AngII (400 ng/kg per minute) in APA-KO and wild-type mice. Although we observed no significant difference in achieved systolic BP, AngII-treated APA-KO mice developed a significant rise in albuminuria not observed in AngII-treated wild-type mice along with increased segmental and global sclerosis and/or collapse of juxtamedullary glomeruli, microcystic tubular dilation, and tubulointerstitial fibrosis. In parallel, AngII treatment significantly increased the kidney AngII content and attenuated the expression of podocyte nephrin in APA-KO mice but not in wild-type controls. These data show that deficiency of APA increases susceptibility to glomerular injury in BALB/c mice. The augmented AngII-mediated kidney injury observed in association with increased intrarenal AngII accumulation in the absence of APA suggests a protective metabolizing role of APA in AngII-mediated glomerular diseases.
Keywords: albuminuria; angiotensin II; glomerulosclerosis; intrarenal; murine; podocyte.
Copyright © 2017 by the American Society of Nephrology.
Figures



Similar articles
-
Hypertension and angiotensin II hypersensitivity in aminopeptidase A-deficient mice.Mol Med. 2003 Jan-Feb;9(1-2):57-62. Mol Med. 2003. PMID: 12765341 Free PMC article.
-
Lack of angiotensin II conversion to angiotensin III increases water but not alcohol consumption in aminopeptidase A-deficient mice.Regul Pept. 2006 Sep 11;136(1-3):130-7. doi: 10.1016/j.regpep.2006.06.001. Epub 2006 Aug 2. Regul Pept. 2006. PMID: 16889841
-
Possible involvement of aminopeptidase A in hypertension and renal damage in Dahl salt-sensitive rats.Am J Hypertens. 2005 Apr;18(4 Pt 1):538-43. doi: 10.1016/j.amjhyper.2004.11.010. Am J Hypertens. 2005. PMID: 15831365
-
Aminopeptidase A, which generates one of the main effector peptides of the brain renin-angiotensin system, angiotensin III, has a key role in central control of arterial blood pressure.Biochem Soc Trans. 2000;28(4):435-40. Biochem Soc Trans. 2000. PMID: 10961935 Review.
-
New insights into the importance of aminopeptidase A in hypertension.Heart Fail Rev. 2008 Sep;13(3):273-84. doi: 10.1007/s10741-007-9065-7. Epub 2007 Nov 8. Heart Fail Rev. 2008. PMID: 17990103 Free PMC article. Review.
Cited by
-
The motor protein Myo1c regulates transforming growth factor-β-signaling and fibrosis in podocytes.Kidney Int. 2019 Jul;96(1):139-158. doi: 10.1016/j.kint.2019.02.014. Epub 2019 Mar 4. Kidney Int. 2019. PMID: 31097328 Free PMC article.
-
Mitochondrial biogenesis induced by the β2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury.Kidney Int. 2019 Sep;96(3):656-673. doi: 10.1016/j.kint.2019.03.023. Epub 2019 May 6. Kidney Int. 2019. PMID: 31262488 Free PMC article.
-
AMPK mediates regulation of glomerular volume and podocyte survival.JCI Insight. 2021 Oct 8;6(19):e150004. doi: 10.1172/jci.insight.150004. JCI Insight. 2021. PMID: 34473647 Free PMC article.
-
Disruption of the exocyst induces podocyte loss and dysfunction.J Biol Chem. 2019 Jun 28;294(26):10104-10119. doi: 10.1074/jbc.RA119.008362. Epub 2019 May 9. J Biol Chem. 2019. PMID: 31073028 Free PMC article.
-
Comparative evaluation of glomerular morphometric techniques reveals differential technical artifacts between focal segmental glomerulosclerosis and normal glomeruli.Physiol Rep. 2023 Jul;11(13):e15688. doi: 10.14814/phy2.15688. Physiol Rep. 2023. PMID: 37423891 Free PMC article.
References
-
- Bauer J, Berthold H, Schaefer F, Ehmke H, Parekh N: Quantification of conversion and degradation of circulating angiotensin in rats. Am J Physiol 277: R412–R418, 1999 - PubMed
-
- Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG: Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 293: F398–F407, 2007 - PubMed
-
- Yang HY, Erdös EG, Chiang TS: New enzymatic route for the inactivation of angiotensin. Nature 218: 1224–1226, 1968 - PubMed
-
- Allred AJ, Diz DI, Ferrario CM, Chappell MC: Pathways for angiotensin-(1---7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol 279: F841–F850, 2000 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

