Temporal dynamics of retinal and extraretinal signals in the FEFsem during smooth pursuit eye movements
- PMID: 28202571
- PMCID: PMC5411476
- DOI: 10.1152/jn.00786.2016
Temporal dynamics of retinal and extraretinal signals in the FEFsem during smooth pursuit eye movements
Abstract
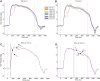
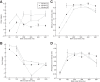
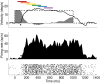
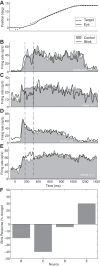
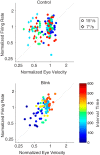
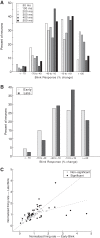
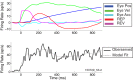
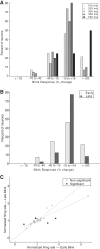
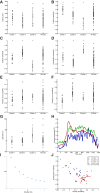
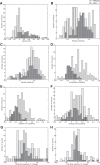
Neurons in the smooth eye movement subregion of the frontal eye field (FEFsem) are known to play an important role in voluntary smooth pursuit eye movements. Underlying this function are projections to parietal and prefrontal visual association areas and subcortical structures, all known to play vital but differing roles in the execution of smooth pursuit. Additionally, the FEFsem has been shown to carry a diverse array of signals (e.g., eye velocity, acceleration, gain control). We hypothesized that distinct subpopulations of FEFsem neurons subserve these diverse functions and projections, and that the relative weights of retinal and extraretinal signals could form the basis for categorization of units. To investigate this, we used a step-ramp tracking task with a target blink to determine the relative contributions of retinal and extraretinal signals in individual FEFsem neurons throughout pursuit. We found that the contributions of retinal and extraretinal signals to neuronal activity and behavior change throughout the time course of pursuit. A clustering algorithm revealed three distinct neuronal subpopulations: cluster 1 was defined by a higher sensitivity to eye velocity, acceleration, and retinal image motion; cluster 2 had greater activity during blinks; and cluster 3 had significantly greater eye position sensitivity. We also performed a comparison with a sample of medial superior temporal neurons to assess similarities and differences between the two areas. Our results indicate the utility of simple tests such as the target blink for parsing the complex and multifaceted roles of cortical areas in behavior.NEW & NOTEWORTHY The frontal eye field (FEF) is known to play a critical role in volitional smooth pursuit, carrying a variety of signals that are distributed throughout the brain. This study used a novel application of a target blink task during step ramp tracking to determine, in combination with a clustering algorithm, the relative contributions of retinal and extraretinal signals to FEF activity and the extent to which these contributions could form the basis for a categorization of neurons.
Keywords: FEF; extraretinal; initiation; smooth pursuit.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Brodal P. The cortical projection to the nucleus reticularis tegmenti pontis in the rhesus monkey. Exp Brain Res 38: 19–27, 1980. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

