Mussel adhesion - essential footwork
- PMID: 28202646
- PMCID: PMC5312731
- DOI: 10.1242/jeb.134056
Mussel adhesion - essential footwork
Abstract
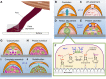
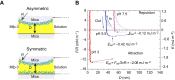
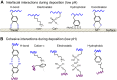
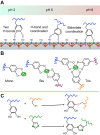
Robust adhesion to wet, salt-encrusted, corroded and slimy surfaces has been an essential adaptation in the life histories of sessile marine organisms for hundreds of millions of years, but it remains a major impasse for technology. Mussel adhesion has served as one of many model systems providing a fundamental understanding of what is required for attachment to wet surfaces. Most polymer engineers have focused on the use of 3,4-dihydroxyphenyl-l-alanine (Dopa), a peculiar but abundant catecholic amino acid in mussel adhesive proteins. The premise of this Review is that although Dopa does have the potential for diverse cohesive and adhesive interactions, these will be difficult to achieve in synthetic homologs without a deeper knowledge of mussel biology; that is, how, at different length and time scales, mussels regulate the reactivity of their adhesive proteins. To deposit adhesive proteins onto target surfaces, the mussel foot creates an insulated reaction chamber with extreme reaction conditions such as low pH, low ionic strength and high reducing poise. These conditions enable adhesive proteins to undergo controlled fluid-fluid phase separation, surface adsorption and spreading, microstructure formation and, finally, solidification.
Keywords: Dopa; Foot behavior; Interfacial chemistry; Mussel foot proteins.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The author declares no competing or financial interests.
Figures



References
-
- Allen J. A., Cook M., Jackson D. J., Preston S. and Worth E. M. (1976). Observations on the rate of production and mechanical properties of the byssus threads of Mytilus edulis. J. Mollusc. Stud. 42, 279-289.
-
- Ashby M. F. (1983). The mechanical properties of cellular solids. Metall. Trans. 14A, 1755-1769. 10.1007/BF02645546 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

