Stress-induced O-GlcNAcylation: an adaptive process of injured cells
- PMID: 28202678
- PMCID: PMC6492270
- DOI: 10.1042/BST20160153
Stress-induced O-GlcNAcylation: an adaptive process of injured cells
Abstract
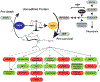
In the 30 years, since the discovery of nucleocytoplasmic glycosylation, O-GlcNAc has been implicated in regulating cellular processes as diverse as protein folding, localization, degradation, activity, post-translational modifications, and interactions. The cell co-ordinates these molecular events, on thousands of cellular proteins, in concert with environmental and physiological cues to fine-tune epigenetics, transcription, translation, signal transduction, cell cycle, and metabolism. The cellular stress response is no exception: diverse forms of injury result in dynamic changes to the O-GlcNAc subproteome that promote survival. In this review, we discuss the biosynthesis of O-GlcNAc, the mechanisms by which O-GlcNAc promotes cytoprotection, and the clinical significance of these data.
Keywords: OGT; chaperone; glycoprotein; heat shock response; mgea5; signal transduction.
© 2017 The Author(s); published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Declarations of interest:
The authors have no conflicts of interest to report.
Figures
References
-
- Nollen EAA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. Journal of Cell Science. 2002;115(Pt 14): 2809–2816. - PubMed
-
- Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. The Journal of biological chemistry. [Online] 2004;279(29): 30133–30142. Available from: doi:10.1074/jbc.M403773200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

