Stretchable living materials and devices with hydrogel-elastomer hybrids hosting programmed cells
- PMID: 28202725
- PMCID: PMC5338509
- DOI: 10.1073/pnas.1618307114
Stretchable living materials and devices with hydrogel-elastomer hybrids hosting programmed cells
Abstract
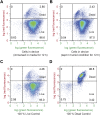
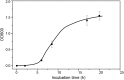
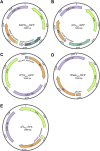
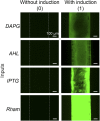
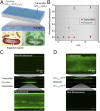
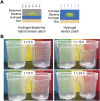
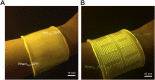
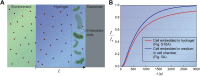
Living systems, such as bacteria, yeasts, and mammalian cells, can be genetically programmed with synthetic circuits that execute sensing, computing, memory, and response functions. Integrating these functional living components into materials and devices will provide powerful tools for scientific research and enable new technological applications. However, it has been a grand challenge to maintain the viability, functionality, and safety of living components in freestanding materials and devices, which frequently undergo deformations during applications. Here, we report the design of a set of living materials and devices based on stretchable, robust, and biocompatible hydrogel-elastomer hybrids that host various types of genetically engineered bacterial cells. The hydrogel provides sustainable supplies of water and nutrients, and the elastomer is air-permeable, maintaining long-term viability and functionality of the encapsulated cells. Communication between different bacterial strains and with the environment is achieved via diffusion of molecules in the hydrogel. The high stretchability and robustness of the hydrogel-elastomer hybrids prevent leakage of cells from the living materials and devices, even under large deformations. We show functions and applications of stretchable living sensors that are responsive to multiple chemicals in a variety of form factors, including skin patches and gloves-based sensors. We further develop a quantitative model that couples transportation of signaling molecules and cellular response to aid the design of future living materials and devices.
Keywords: biochemical sensors; genetically engineered bacteria; hydrogels; synthetic biology; wearable devices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Siuti P, Yazbek J, Lu TK. Engineering genetic circuits that compute and remember. Nat Protoc. 2014;9(6):1292–1300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

