Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection
- PMID: 28202771
- PMCID: PMC5678930
- DOI: 10.1126/scitranslmed.aag1809
Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection
Abstract
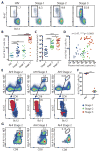
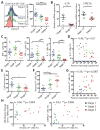
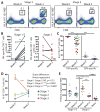
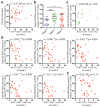
CD8+ T cells play a critical role in controlling HIV viremia and could be important in reducing HIV-infected cells in approaches to eradicate HIV. The simian immunodeficiency virus model provided the proof of concept for a CD8+ T cell-mediated reservoir clearance but showed conflicting evidence on the role of these cells to eliminate HIV-infected cells. In humans, HIV-specific CD8+ T cell responses have not been associated with a reduction of the HIV-infected cell pool in vivo. We studied HIV-specific CD8+ T cells in the RV254 cohort of individuals initiating ART in the earliest stages of acute HIV infection (AHI). We showed that the HIV-specific CD8+ T cells generated as early as AHI stages 1 and 2 before peak viremia are delayed in expanding and acquiring effector functions but are endowed with higher memory potential. In contrast, the fully differentiated HIV-specific CD8+ T cells at peak viremia in AHI stage 3 were more prone to apoptosis but were associated with a steeper viral load decrease after ART initiation. Their capacity to persist in vivo after ART initiation correlated with a lower HIV DNA reservoir. These findings demonstrate that HIV-specific CD8+ T cell magnitude and differentiation are delayed in the earliest stages of infection. These results also demonstrate that potent HIV-specific CD8+ T cells contribute to the reduction of the pool of HIV-producing cells and the HIV reservoir seeding in vivo and provide the rationale to design interventions aiming at inducing these potent responses to cure HIV infection.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures

Comment in
-
Very early antiretroviral therapy permits CD8 T cells to keep HIV reservoirs at bay.Ann Transl Med. 2017 Nov;5(21):434. doi: 10.21037/atm.2017.08.38. Ann Transl Med. 2017. PMID: 29201886 Free PMC article. No abstract available.
References
-
- McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. - PubMed
-
- Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–2714. - PMC - PubMed
-
- Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, Piechocka-Trocha A, Cesa KT, Sela J, Cung TD, Toth I, Pereyra F, Yu XG, Douek DC, Kaufmann DE, Allen TM, Walker BD. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nature. 2012;13:691–700. - PMC - PubMed
-
- Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, 3rd, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr, Haase AT, Lifson JD, Allen TM, Watkins DI. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

