Discovery of Novel Pneumococcal Serotype 35D, a Natural WciG-Deficient Variant of Serotype 35B
- PMID: 28202800
- PMCID: PMC5405259
- DOI: 10.1128/JCM.00054-17
Discovery of Novel Pneumococcal Serotype 35D, a Natural WciG-Deficient Variant of Serotype 35B
Abstract
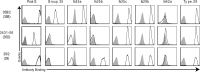
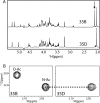
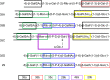
Pneumococcus (Streptococcus pneumoniae) remains a significant cause of morbidity and mortality, especially among those at the extremes of age. Its capsular polysaccharide is essential for systemic virulence. Over 90 serologically distinct pneumococcal capsular polysaccharides (serotypes) are recognized, but they are unequal in prevalence. Because antibodies against the capsule are protective, polysaccharide conjugate vaccines, which are constructed against the most prevalent serotypes, have caused great reductions in pneumococcal disease caused by these serotypes. In response, however, the relative prevalences of serotypes have shifted. Certain previously rare serotypes, such as serotype 35B, are increasing in prevalence. Serotype 35B is thus a likely future vaccine candidate, but due to their previous rarity, serotype 35B strains have not been scrutinized for underlying heterogeneity. We studied putative serotype 35B clinical isolates to assess the uniformity of their serological reactions. While most isolates exhibited the accepted serology of serotype 35B, one isolate failed to bind to critical serotyping reagents. We determined that the genetic basis for this aberrant serology was the presence of inactivating mutations in the O-acetyltransferase gene wciG Complementation studies in a wciG deletion strain verified that the mutant WciG was nonfunctional, and the serology of the mutant could be restored through complementation with a construct encoding a functional WciG. Nuclear magnetic resonance studies confirmed that the capsule of the WciG-deficient isolate lacked O-acetylation but was otherwise identical to serotype 35B. As this isolate expresses a unique serology with unique biochemistry and a stable genetic basis, we named its novel capsule serotype 35D.
Keywords: O-acetylation; Streptococcus pneumoniae; capsular polysaccharide; serogroup 35; serotype 35B; serotype 35D.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR, Active Bacterial Core surveillance/Emerging Infections Program Network. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201:32–41. doi: 10.1086/648593. - DOI - PubMed
-
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, Miller L, Scherzinger K, Thomas A, Farley MM, Zell ER, Taylor TH Jr, Pondo T, Rodgers L, McGee L, Beall B, Jorgensen JH, Whitney CG. 2015. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 15:301–309. doi: 10.1016/S1473-3099(14)71081-3. - DOI - PMC - PubMed
-
- Sharma D, Baughman W, Holst A, Thomas S, Jackson D, da Gloria Carvalho M, Beall B, Satola S, Jerris R, Jain S, Farley MM, Nuorti JP. 2013. Pneumococcal carriage and invasive disease in children before introduction of the 13-valent conjugate vaccine: comparison with the era before 7-valent conjugate vaccine. Pediatr Infect Dis J 32:196. doi: 10.1097/INF.0b013e3182788fdd. - DOI - PubMed
-
- Neufeld F, Haendel L. 1910. Weitere untersuchungen uber pneumokokken-heilsera. III. Mitteilung Arbeiten aus dem Kaiserlichen Gesundheitsamte 34:293–304.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

