Calpain-1 deletion impairs mGluR-dependent LTD and fear memory extinction
- PMID: 28202907
- PMCID: PMC5311935
- DOI: 10.1038/srep42788
Calpain-1 deletion impairs mGluR-dependent LTD and fear memory extinction
Abstract
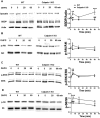
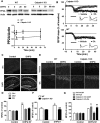
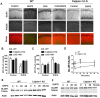
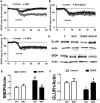
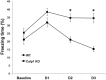
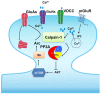
Recent studies indicate that calpain-1 is required for the induction of long-term potentiation (LTP) elicited by theta-burst stimulation in field CA1 of hippocampus. Here we determined the contribution of calpain-1 in another type of synaptic plasticity, the long-term depression (LTD) elicited by activation of type-I metabotropic glutamate receptors (mGluR-LTD). mGluR-LTD was associated with calpain-1 activation following T-type calcium channel opening, and resulted in the truncation of a regulatory subunit of PP2A, B56α. This signaling pathway was required for both the early and late phase of Arc translation during mGluR-LTD, through a mechanism involving mTOR and ribosomal protein S6 activation. In contrast, in hippocampal slices from calpain-1 knock-out (KO) mice, application of the mGluR agonist, DHPG, did not result in B56α truncation, increased Arc synthesis and reduced levels of membrane GluA1-containing AMPA receptors. Consistently, mGluR-LTD was impaired in calpain-1 KO mice, and the impairment could be rescued by phosphatase inhibitors, which also restored Arc translation in response to DHPG. Furthermore, calpain-1 KO mice exhibited impairment in fear memory extinction to tone presentation. These results indicate that calpain-1 plays a critical role in mGluR-LTD and is involved in many forms of synaptic plasticity and learning and memory.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression.J Neurosci. 2012 Apr 25;32(17):5924-36. doi: 10.1523/JNEUROSCI.4650-11.2012. J Neurosci. 2012. PMID: 22539853 Free PMC article.
-
Impaired cerebellar plasticity and eye-blink conditioning in calpain-1 knock-out mice.Neurobiol Learn Mem. 2020 Apr;170:106995. doi: 10.1016/j.nlm.2019.02.005. Epub 2019 Feb 5. Neurobiol Learn Mem. 2020. PMID: 30735788 Review.
-
Reelin Regulates Neuronal Excitability through Striatal-Enriched Protein Tyrosine Phosphatase (STEP61) and Calcium Permeable AMPARs in an NMDAR-Dependent Manner.J Neurosci. 2021 Sep 1;41(35):7340-7349. doi: 10.1523/JNEUROSCI.0388-21.2021. Epub 2021 Jul 21. J Neurosci. 2021. PMID: 34290083 Free PMC article.
-
ArcKR expression modifies synaptic plasticity following epileptic activity: Differential effects with in vitro and in vivo seizure-induction protocols.Epilepsia. 2024 Jul;65(7):2152-2164. doi: 10.1111/epi.17981. Epub 2024 May 28. Epilepsia. 2024. PMID: 38804501
-
Molecular mechanisms coordinating functional and morphological plasticity at the synapse: role of GluA2/N-cadherin interaction-mediated actin signaling in mGluR-dependent LTD.Cell Signal. 2013 Feb;25(2):397-402. doi: 10.1016/j.cellsig.2012.11.007. Epub 2012 Nov 12. Cell Signal. 2013. PMID: 23153583 Review.
Cited by
-
Botanicals as modulators of depression and mechanisms involved.Chin Med. 2019 Jul 15;14:24. doi: 10.1186/s13020-019-0246-9. eCollection 2019. Chin Med. 2019. PMID: 31338119 Free PMC article. Review.
-
Depichering the Effects of Astragaloside IV on AD-Like Phenotypes: A Systematic and Experimental Investigation.Oxid Med Cell Longev. 2021 Sep 24;2021:1020614. doi: 10.1155/2021/1020614. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34616501 Free PMC article.
-
Abnormal response to chronic social defeat stress and fear extinction in a mouse model of Lynx2-based cholinergic dysregulation.Front Neurosci. 2025 Apr 1;19:1466166. doi: 10.3389/fnins.2025.1466166. eCollection 2025. Front Neurosci. 2025. PMID: 40236946 Free PMC article.
-
Role of the TRPC1 Channel in Hippocampal Long-Term Depression and in Spatial Memory Extinction.Int J Mol Sci. 2020 Mar 3;21(5):1712. doi: 10.3390/ijms21051712. Int J Mol Sci. 2020. PMID: 32138218 Free PMC article.
-
Novel Roles of the GPI-Anchor Cleaving Enzyme, GDE2, in Hippocampal Synaptic Morphology and Function.eNeuro. 2025 Jul 29;12(7):ENEURO.0102-25.2025. doi: 10.1523/ENEURO.0102-25.2025. Print 2025 Jul. eNeuro. 2025. PMID: 40634123 Free PMC article.
References
-
- Neves G., Cooke S. F. & Bliss T. V. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci. 9, 65–75 (2008). - PubMed
-
- Sweatt J. D. Neural Plasticity & Behavior - Sixty Years of Conceptual Advances. J. Neurochem. Epub ahead of print (2016). - PubMed
-
- Kim J. et al.. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem. Biophys. Res. Comm. 355, 188–193 (2007). - PubMed
-
- Dalton G. L., Wang Y. T., Floresco S. B. & Phillips A. G. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology 33, 2416–2426 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

