Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer
- PMID: 28203075
- PMCID: PMC5293363
- DOI: 10.2147/IJN.S115136
Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer
Abstract
Background: Cervical cancer is a major world health problem for women. Currently, cancer research focuses on improving therapy for cervical cancer using various treatment options such as co-delivery of chemotherapeutic agents by nanocarriers.
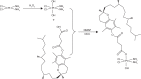
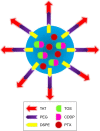
Purpose: The aim of this study was to develop trans-activating transcriptional activator (TAT)-modified solid lipid nanoparticles (SLNs) for co-delivery of paclitaxel (PTX) and α-tocopherol succinate-cisplatin prodrug (TOS-CDDP) (TAT PTX/TOS-CDDP SLNs) in order to achieve synergistic antitumor activity against cervical cancer.
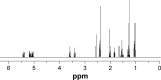
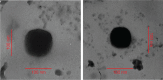
Methods: Lipid prodrug of CDDP (TOS-CDDP) and TAT-containing polyethylene glycol-distearoyl-phosphatidylethanolamine (TAT-PEG-DSPE) were synthesized. TAT PTX/TOS-CDDP SLNs were prepared by emulsification and solvent evaporation method. Physicochemical characteristics of SLNs such as size, morphology, and release profiles were explored. In vitro and in vivo studies were carried out to assess the efficacy of their antitumor activity in target cells.
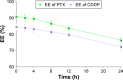
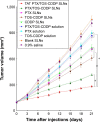
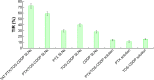
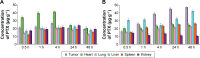
Results: TAT PTX/TOS-CDDP SLNs could be successfully internalized by HeLa cells and showed a synergistic effect in the suppression of cervical tumor cell growth. They exhibited high tumor tissue accumulation, superior antitumor efficiency, and much lower toxicity in vivo.
Conclusion: The present study indicates that the co-delivery system provides a promising platform as a combination therapy for the treatment of cervical cancer, and possibly other types of cancer as well.
Keywords: cell-penetrating peptide; cervical cancer; combination therapy; lipid-based prodrug; solid lipid nanoparticles.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





Similar articles
-
RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy.Biomed Pharmacother. 2018 Oct;106:275-284. doi: 10.1016/j.biopha.2018.06.137. Epub 2018 Jun 28. Biomed Pharmacother. 2018. PMID: 29966971
-
Co-delivery of cisplatin and paclitaxel by folic acid conjugated amphiphilic PEG-PLGA copolymer nanoparticles for the treatment of non-small lung cancer.Oncotarget. 2015 Dec 8;6(39):42150-68. doi: 10.18632/oncotarget.6243. Oncotarget. 2015. PMID: 26517524 Free PMC article.
-
α-Tocopherol Succinate-Anchored PEGylated Poly(amidoamine) Dendrimer for the Delivery of Paclitaxel: Assessment of in Vitro and in Vivo Therapeutic Efficacy.Mol Pharm. 2019 Apr 1;16(4):1541-1554. doi: 10.1021/acs.molpharmaceut.8b01232. Epub 2019 Mar 12. Mol Pharm. 2019. PMID: 30817166
-
Update on the Use of Nanocarriers and Drug Delivery Systems and Future Directions in Cervical Cancer.J Immunol Res. 2022 May 4;2022:1636908. doi: 10.1155/2022/1636908. eCollection 2022. J Immunol Res. 2022. PMID: 35571568 Free PMC article. Review.
-
Solid Lipid Nanoparticles, an Alternative for the Treatment of Triple-Negative Breast Cancer.Int J Mol Sci. 2024 Oct 5;25(19):10712. doi: 10.3390/ijms251910712. Int J Mol Sci. 2024. PMID: 39409041 Free PMC article. Review.
Cited by
-
Targeted Therapy for Hepatocellular Carcinoma: Co-Delivery of Sorafenib and Curcumin Using Lactosylated pH-Responsive Nanoparticles.Drug Des Devel Ther. 2020 Feb 18;14:647-659. doi: 10.2147/DDDT.S238955. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32109990 Free PMC article.
-
Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence.Br J Pharmacol. 2020 Mar;177(6):1409-1423. doi: 10.1111/bph.14816. Epub 2019 Nov 27. Br J Pharmacol. 2020. PMID: 31368509 Free PMC article. Review.
-
Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs' Dosage Ratio Effect.Molecules. 2019 Mar 15;24(6):1035. doi: 10.3390/molecules24061035. Molecules. 2019. PMID: 30875934 Free PMC article. Review.
-
Low dimensional nanomaterials for treating acute kidney injury.J Nanobiotechnology. 2022 Dec 1;20(1):505. doi: 10.1186/s12951-022-01712-2. J Nanobiotechnology. 2022. PMID: 36456976 Free PMC article. Review.
-
Traditional Chinese medicine-combination therapies utilizing nanotechnology-based targeted delivery systems: a new strategy for antitumor treatment.Int J Nanomedicine. 2019 Mar 22;14:2029-2053. doi: 10.2147/IJN.S197889. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30962686 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. - PubMed
-
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. - PubMed
-
- Cervical Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012 International Agency for Research on Cancer and World Health Organization. 2012. [Accessed March 16, 2016]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
-
- Hani U, Osmani RA, Bhosale RR, Shivakumar HG, Kulkarni PK. Current perspectives on novel drug delivery systems and approaches for management of cervical cancer: a comprehensive review. Curr Drug Targets. 2016;17(3):337–352. - PubMed
-
- Vivero-Escoto JL, Slowing II, Lin VS. Tuning the cellular uptake and cytotoxicity properties of oligonucleotide intercalator-functionalized mesoporous silica nanoparticles with human cervical cancer cells HeLa. Biomaterials. 2010;31(6):1325–1333. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

