Regulation of Sterol Biosynthesis in the Human Fungal Pathogen Aspergillus fumigatus: Opportunities for Therapeutic Development
- PMID: 28203225
- PMCID: PMC5285346
- DOI: 10.3389/fmicb.2017.00092
Regulation of Sterol Biosynthesis in the Human Fungal Pathogen Aspergillus fumigatus: Opportunities for Therapeutic Development
Abstract
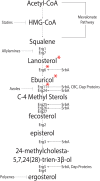
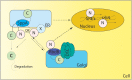
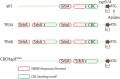
Sterols are a major component of eukaryotic cell membranes. For human fungal infections caused by the filamentous fungus Aspergillus fumigatus, antifungal drugs that target sterol biosynthesis and/or function remain the standard of care. Yet, an understanding of A. fumigatus sterol biosynthesis regulatory mechanisms remains an under developed therapeutic target. The critical role of sterol biosynthesis regulation and its interactions with clinically relevant azole drugs is highlighted by the basic helix loop helix (bHLH) class of transcription factors known as Sterol Regulatory Element Binding Proteins (SREBPs). SREBPs regulate transcription of key ergosterol biosynthesis genes in fungi including A. fumigatus. In addition, other emerging regulatory pathways and target genes involved in sterol biosynthesis and drug interactions provide additional opportunities including the unfolded protein response, iron responsive transcriptional networks, and chaperone proteins such as Hsp90. Thus, targeting molecular pathways critical for sterol biosynthesis regulation presents an opportunity to improve therapeutic options for the collection of diseases termed aspergillosis. This mini-review summarizes our current understanding of sterol biosynthesis regulation with a focus on mechanisms of transcriptional regulation by the SREBP family of transcription factors.
Keywords: Aspergillus fumigatus; SREBPs; antifungal agents; ergosterol; triazoles.
Figures
References
-
- Alcazar-Fuoli L., Mellado E., Garcia-Effron G., Buitrago M. J., Lopez J. F., Grimalt J. O., et al. (2006). Aspergillus fumigatus C-5 sterol desaturases Erg3A and Erg3B: role in sterol biosynthesis and antifungal drug susceptibility. Antimicrob. Agents Chemother. 50 453–460. 10.1128/AAC.50.2.453-460.2006 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

