Activation of the Wnt Pathway by Mycobacterium tuberculosis: A Wnt-Wnt Situation
- PMID: 28203237
- PMCID: PMC5285348
- DOI: 10.3389/fimmu.2017.00050
Activation of the Wnt Pathway by Mycobacterium tuberculosis: A Wnt-Wnt Situation
Abstract
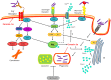
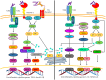
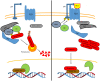
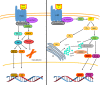
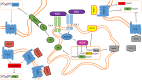
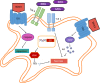
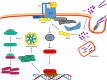
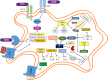
Mycobacterium tuberculosis (M. tuberculosis), an intracellular pathogenic Gram-positive bacterium, is the cause of tuberculosis (TB), a major worldwide human infectious disease. The innate immune system is the first host defense against M. tuberculosis. The recognition of this pathogen is mediated by several classes of pattern recognition receptors expressed on the host innate immune cells, including Toll-like receptors, Nod-like receptors, and C-type lectin receptors like Dectin-1, the Mannose receptor, and DC-SIGN. M. tuberculosis interaction with any of these receptors activates multiple signaling pathways among which the protein kinase C, the MAPK, and the NFκB pathways have been widely studied. These pathways have been implicated in macrophage invasion, M. tuberculosis survival, and impaired immune response, thus promoting a successful infection and disease. Interestingly, the Wnt signaling pathway, classically regarded as a pathway involved in the control of cell proliferation, migration, and differentiation in embryonic development, has recently been involved in immunoregulatory mechanisms in infectious and inflammatory diseases, such as TB, sepsis, psoriasis, rheumatoid arthritis, and atherosclerosis. In this review, we present the current knowledge supporting a role for the Wnt signaling pathway during macrophage infection by M. tuberculosis and the regulation of the immune response against M. tuberculosis. Understanding the cross talk between different signaling pathways activated by M. tuberculosis will impact on the search for new therapeutic targets to fuel the rational design of drugs aimed to restore the immunological response against M. tuberculosis.
Keywords: Wnt signaling; immune response; inflammation; macrophage infection; microRNAs; tuberculosis.
Figures
Similar articles
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs.Curr Opin Infect Dis. 2008 Jun;21(3):279-86. doi: 10.1097/QCO.0b013e3282f88b5d. Curr Opin Infect Dis. 2008. PMID: 18448973 Review.
-
Innate immune recognition of Mycobacterium tuberculosis.Clin Dev Immunol. 2011;2011:405310. doi: 10.1155/2011/405310. Epub 2011 Apr 7. Clin Dev Immunol. 2011. PMID: 21603213 Free PMC article. Review.
-
Partial redundancy of the pattern recognition receptors, scavenger receptors, and C-type lectins for the long-term control of Mycobacterium tuberculosis infection.J Immunol. 2010 Jun 15;184(12):7057-70. doi: 10.4049/jimmunol.1000164. Epub 2010 May 19. J Immunol. 2010. PMID: 20488784
-
Innate Immune Pattern Recognition Receptors of Mycobacterium tuberculosis: Nature and Consequences for Pathogenesis of Tuberculosis.Adv Exp Med Biol. 2021;1313:179-215. doi: 10.1007/978-3-030-67452-6_9. Adv Exp Med Biol. 2021. PMID: 34661896
Cited by
-
The emerging role of Wnt5a in the promotion of a pro-inflammatory and immunosuppressive tumor microenvironment.Cancer Metastasis Rev. 2020 Sep;39(3):933-952. doi: 10.1007/s10555-020-09878-7. Cancer Metastasis Rev. 2020. PMID: 32435939 Review.
-
The Bidirectional Relationship between Pulmonary Tuberculosis and Lung Cancer.Int J Environ Res Public Health. 2023 Jan 10;20(2):1282. doi: 10.3390/ijerph20021282. Int J Environ Res Public Health. 2023. PMID: 36674038 Free PMC article. Review.
-
Wnt5A Signaling Promotes Defense Against Bacterial Pathogens by Activating a Host Autophagy Circuit.Front Immunol. 2018 Apr 9;9:679. doi: 10.3389/fimmu.2018.00679. eCollection 2018. Front Immunol. 2018. PMID: 29686674 Free PMC article.
-
Distinct Roles of Wnt/β-Catenin Signaling in the Pathogenesis of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis.Mediators Inflamm. 2017;2017:3520581. doi: 10.1155/2017/3520581. Epub 2017 May 9. Mediators Inflamm. 2017. PMID: 28588349 Free PMC article. Review.
-
Ca2+ as a therapeutic target in cancer.Adv Cancer Res. 2020;148:233-317. doi: 10.1016/bs.acr.2020.05.003. Epub 2020 Jul 9. Adv Cancer Res. 2020. PMID: 32723565 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

