The renal effects of SGLT2 inhibitors and a mini-review of the literature
- PMID: 28203358
- PMCID: PMC5298360
- DOI: 10.1177/2042018816676239
The renal effects of SGLT2 inhibitors and a mini-review of the literature
Abstract
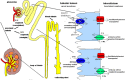
Sodium-glucose linked transporter 2 (SGLT2) inhibitors are a new and promising class of antidiabetic agents which target renal tubular glucose reabsorption. Their action is based on the blockage of SGLT2 sodium-glucose cotransporters that are located at the luminal membrane of tubular cells of the proximal convoluted tubule, inducing glucosuria. It has been proven that they significantly reduce glycated hemoglobin (HbA1c), along with fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus (T2DM). The glucosuria-induced caloric loss as well as the osmotic diuresis significantly decrease body weight and blood pressure, respectively. Given that SGLT2 inhibitors do not interfere with insulin action and secretion, their efficacy is sustained despite the progressive β-cell failure in T2DM. They are well tolerated, with a low risk of hypoglycemia. Their most frequent adverse events are minor: genital and urinal tract infections. Recently, it was demonstrated that empagliflozin presents a significant cardioprotective effect. Although the SGLT2 inhibitors' efficacy is affected by renal function, new data have been presented that some SGLT2 inhibitors, even in mild and moderate renal impairment, induce significant HbA1c reduction. Moreover, recent data indicate that SGLT2 inhibition has a beneficial renoprotective effect. The role of this review paper is to explore the current evidence on the renal effects of SGLT2 inhibitors.
Keywords: SGLT transporters; SGLT2 inhibitors; albuminuria; glucosuria; hyperfiltration; renal impairment; renoprotection; tubulointerstitial fibrosis; type 2 diabetes.
Conflict of interest statement
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: John Doupis has received honoraria for lecturing on SGLT2 inhibitors, from AstraZeneca, Janssen, and Boehringer Ingelheim (Ingelheim, Germany). The other authors have no conflict of interest to disclose.
Figures
References
-
- Abdul-Ghani M., DeFronzo R. (2008) Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 14: 782–790. - PubMed
-
- Astra Zeneca. (2012) Forxiga™ (dapagliflozin) now approved in European Union for treatment of type 2 diabetes. Available at: https://www.astrazeneca.com/media-centre/press-releases/2012/FORXIGA-dap... (accessed 28 October 2016).
-
- Augustin R. (2010) The protein family of glucose transport facilitators: it’s not only about glucose after all: critical review. IUBMB Life 62: 315–333. - PubMed
-
- Bailey C., Gross J., Pieters A., Bastien A., List J. (2010) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223–2233. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

