Efficacy and safety of ClairYg®, a ready-to-use intravenous immunoglobulin, in adult patients with primary immune thrombocytopenia
- PMID: 28203488
- PMCID: PMC5306448
Efficacy and safety of ClairYg®, a ready-to-use intravenous immunoglobulin, in adult patients with primary immune thrombocytopenia
Erratum in
-
Erratum: Efficacy and safety of ClairYg®, a ready-to-use intravenous immunoglobulin, in adult patients with primary immune thrombocytopenia.Am J Blood Res. 2017 Jun 15;7(3):29. eCollection 2017. Am J Blood Res. 2017. PMID: 28690918 Free PMC article.
Abstract
Purpose: The present study was designed to assess the efficacy and safety of IGNG that is a new liquid, saccharose and maltose-free highly purified ready-to-use 5% intravenous immunoglobulin (IVIg), in primary immune thrombocytopenic patients with severe thrombocytopenia.
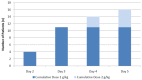
Methods: Nineteen adults with a platelet count ≤ 25 × 109/L received a single dose of IGNG (1 g/kg) on Day 1, with a second identical dose on Day 3 if needed. Patients were followed for 30 days. Primary endpoint was the response rate, defined as the proportion of patients with a platelet count ≥ 50 × 109/L within 96 hours after the first IGNG dose.
Results: All but one of the 17 evaluable patients for efficacy responded with an overall response rate of 94.1% (95% CI 71.3%-99.9%). Response was observed after only one infusion (1 g/kg boby weight) in 11 patients (59%) and the others required a second dose. Mean time to response was 2.2 days. Maximum platelet count was reached within 1 week after the first dose and lasted for approximately 2 weeks. Patients requiring a second dose had lower platelet counts at baseline than patients requiring a single dose. In the 19 evaluable patients for safety, IGNG demonstrated good safety, good hepatic and renal tolerance, and did not induce hemolysis. This trial was registered at the French Medical Agency (AFSSAPS) as #DI n°060735.
Keywords: Intravenous immunoglobulin; efficacy; primary immune thrombocytopenia; safety.
Figures

Similar articles
-
A multicentre, prospective, non-randomized, sequential, open-label trial to demonstrate the bioequivalence between intravenous immunoglobulin new generation (IGNG) and standard IV immunoglobulin (IVIG) in adult patients with primary immunodeficiency (PID).Rev Med Interne. 2017 Sep;38(9):578-584. doi: 10.1016/j.revmed.2017.05.009. Epub 2017 Jul 3. Rev Med Interne. 2017. PMID: 28683953 Clinical Trial.
-
Efficacy and Safety of IQYMUNE®, a Ten Percent Intravenous Immunoglobulin in Adult Patients With Chronic, Primary Immune Thrombocytopenia.J Hematol. 2018 Sep;7(3):87-95. doi: 10.14740/jh385w. Epub 2018 Sep 1. J Hematol. 2018. PMID: 32300420 Free PMC article.
-
Randomized trial of anti-D immunoglobulin versus low-dose intravenous immunoglobulin in the treatment of childhood chronic idiopathic thrombocytopenic purpura.Acta Haematol. 2006;115(1-2):46-52. doi: 10.1159/000089465. Acta Haematol. 2006. PMID: 16424649 Clinical Trial.
-
Efficacy and Safety of Anti-D Immunoglobulins versus Intravenous Immunoglobulins for Immune Thrombocytopenia in Children: Systematic Review and Meta-analysis of Randomized Controlled Trials.J Pediatr. 2019 Jan;204:225-233.e8. doi: 10.1016/j.jpeds.2018.07.065. Epub 2018 Oct 9. J Pediatr. 2019. PMID: 30314658
-
The efficacy of different dose intravenous immunoglobulin in treating acute idiopathic thrombocytopenic purpura: a meta-analysis of 13 randomized controlled trials.Blood Coagul Fibrinolysis. 2010 Dec;21(8):713-21. doi: 10.1097/MBC.0b013e3283401490. Blood Coagul Fibrinolysis. 2010. PMID: 20962624 Review.
Cited by
-
Real-world experience with CLAIRYG® 50 mg/mL (intravenous immunoglobulin) in children under 12 years with primary immunodeficiency or immmune thrombocytopenia: a post-approval safety study.Front Pediatr. 2023 Oct 2;11:1260296. doi: 10.3389/fped.2023.1260296. eCollection 2023. Front Pediatr. 2023. PMID: 37849499 Free PMC article.
References
-
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, hong BH, Cines DB, Gernsheimer TB, Godeau B, Grainger J, Greer I, Hunt BJ, Imbach PA, Lyons G, McMillan R, Rodeghiero F, Sanz MA, Tarantino M, Watson S, Young J, Kuter DJ. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–86. - PubMed
-
- Neunert CE, editor. Current management of immune thrombocytopenia. ASH education book December 6, 2013; 1: 276-282. doi: 10.1182/asheducation-2013.1.276. Available at: http://asheducationbook.hematologylibrary.org/content/2013/1/276.full.pd.... - PubMed
-
- Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost. 2008;99:4–13. - PubMed
-
- Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP) Blood. 2005;106:2244–51. - PubMed
-
- Schoonen WM, Kucera G, Coalson J, Li L, Rutstein M, Mowat F, Fryzek J, Kaye JA. Epidemiology of immune thrombocytopenic purpura in the general practice research database. Br J Haematol. 2009;145:235–44. - PubMed
LinkOut - more resources
Full Text Sources
