Three-dimensional (3D) printing of mouse primary hepatocytes to generate 3D hepatic structure
- PMID: 28203553
- PMCID: PMC5309179
- DOI: 10.4174/astr.2017.92.2.67
Three-dimensional (3D) printing of mouse primary hepatocytes to generate 3D hepatic structure
Abstract
Purpose: The major problem in producing artificial livers is that primary hepatocytes cannot be cultured for many days. Recently, 3-dimensional (3D) printing technology draws attention and this technology regarded as a useful tool for current cell biology. By using the 3D bio-printing, these problems can be resolved.
Methods: To generate 3D bio-printed structures (25 mm × 25 mm), cells-alginate constructs were fabricated by 3D bio-printing system. Mouse primary hepatocytes were isolated from the livers of 6-8 weeks old mice by a 2-step collagenase method. Samples of 4 × 107 hepatocytes with 80%-90% viability were printed with 3% alginate solution, and cultured with well-defined culture medium for primary hepatocytes. To confirm functional ability of hepatocytes cultured on 3D alginate scaffold, we conducted quantitative real-time polymerase chain reaction and immunofluorescence with hepatic marker genes.
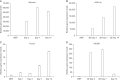
Results: Isolated primary hepatocytes were printed with alginate. The 3D printed hepatocytes remained alive for 14 days. Gene expression levels of Albumin, HNF-4α and Foxa3 were gradually increased in the 3D structures. Immunofluorescence analysis showed that the primary hepatocytes produced hepatic-specific proteins over the same period of time.
Conclusion: Our research indicates that 3D bio-printing technique can be used for long-term culture of primary hepatocytes. It can therefore be used for drug screening and as a potential method of producing artificial livers.
Keywords: Culture; Hepatocytes; Maintenance; Three-dimensional printing.
Conflict of interest statement
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Figures



Similar articles
-
Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures.Organogenesis. 2018 Jan 2;14(1):1-12. doi: 10.1080/15476278.2018.1423931. Epub 2018 Feb 1. Organogenesis. 2018. PMID: 29359998 Free PMC article.
-
Three-Dimensional Bioprinting of Hepatic Structures with Directly Converted Hepatocyte-Like Cells.Tissue Eng Part A. 2018 Apr;24(7-8):576-583. doi: 10.1089/ten.TEA.2017.0161. Epub 2018 Jan 16. Tissue Eng Part A. 2018. PMID: 28726547
-
Investigation of cell viability and morphology in 3D bio-printed alginate constructs with tunable stiffness.J Biomed Mater Res A. 2017 Apr;105(4):1009-1018. doi: 10.1002/jbm.a.35971. Epub 2017 Feb 1. J Biomed Mater Res A. 2017. PMID: 27935198
-
3D-printed patient-specific applications in orthopedics.Orthop Res Rev. 2016 Oct 14;8:57-66. doi: 10.2147/ORR.S99614. eCollection 2016. Orthop Res Rev. 2016. PMID: 30774470 Free PMC article. Review.
-
Review fantastic medical implications of 3D-printing in liver surgeries, liver regeneration, liver transplantation and drug hepatotoxicity testing: A review.Int J Surg. 2018 Aug;56:1-6. doi: 10.1016/j.ijsu.2018.06.004. Epub 2018 Jun 7. Int J Surg. 2018. PMID: 29886280 Review.
Cited by
-
Emulating Human Tissues and Organs: A Bioprinting Perspective Toward Personalized Medicine.Chem Rev. 2020 Oct 14;120(19):11128-11174. doi: 10.1021/acs.chemrev.0c00342. Epub 2020 Sep 16. Chem Rev. 2020. PMID: 32937071 Free PMC article. Review.
-
Biomaterials Based on Marine Resources for 3D Bioprinting Applications.Mar Drugs. 2019 Sep 28;17(10):555. doi: 10.3390/md17100555. Mar Drugs. 2019. PMID: 31569366 Free PMC article. Review.
-
Hydrogels for Liver Tissue Engineering.Bioengineering (Basel). 2019 Jul 5;6(3):59. doi: 10.3390/bioengineering6030059. Bioengineering (Basel). 2019. PMID: 31284412 Free PMC article. Review.
-
Out of Box Thinking to Tangible Science: A Benchmark History of 3D Bio-Printing in Regenerative Medicine and Tissues Engineering.Life (Basel). 2023 Apr 5;13(4):954. doi: 10.3390/life13040954. Life (Basel). 2023. PMID: 37109483 Free PMC article. Review.
-
Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering.Gels. 2024 Mar 22;10(4):216. doi: 10.3390/gels10040216. Gels. 2024. PMID: 38667635 Free PMC article. Review.
References
-
- Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. Mouse liver cell culture. I. Hepatocyte isolation. In Vitro. 1981;17:913–925. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

